Differential spatial representation of taste modalities in the rat gustatory cortex
- PMID: 17287514
- PMCID: PMC6673570
- DOI: 10.1523/JNEUROSCI.5188-06.2007
Differential spatial representation of taste modalities in the rat gustatory cortex
Abstract
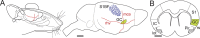
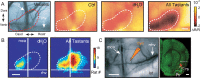
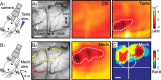
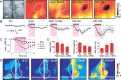
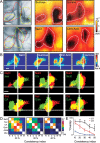
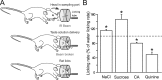
Discrimination between foods is crucial for the nutrition and survival of animals. Remarkable progress has been made through molecular and genetic manipulations in the understanding of the coding of taste at the receptor level. However, much less is known about the cortical processing of taste sensation and the organizing principles of the gustatory cortex (GC). Using genetic tracing, it has recently been shown that sweet and bitter taste are processed through segregated neuronal circuitries along the gustatory pathway up to the cortical level. This is in disagreement with the evidence that GC neurons recorded in both anesthetized and behaving animals responded to multiple taste modalities (including sweet and bitter). To investigate the functional architecture of the GC in regard to taste modalities, we used in vivo intrinsic optical imaging, a technique that has been successfully applied to explore the organization of other neocortical regions. We found that four of the primary taste modalities (sweet, bitter, salty, and sour) are represented by distinctive spatial patterns but that no region was specific to a single modality. In addition, we found that two tastants of similar hedonic value (pleasant or unpleasant) activated areas with more common regions than two tastants with opposite hedonic value. In summary, we propose that these specific cortical patterns can be used to discriminate among various tastants.
Figures
References
-
- Adolphs R, Tranel D, Koenigs M, Damasio AR. Preferring one taste over another without recognizing either. Nat Neurosci. 2005;8:860–861. - PubMed
-
- Bakin JS, Kwon MC, Masino SA, Weinberger NM, Frostig RD. Suprathreshold auditory cortex activation visualized by intrinsic signal optical imaging. Cereb Cortex. 1996;6:120–130. - PubMed
-
- Bartoshuk LM. NaCl thresholds in man: thresholds for water taste or NaCl taste. J Comp Physiol Psychol. 1974;87:310–325. - PubMed
-
- Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
