Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator
- PMID: 17287524
- PMCID: PMC2575670
- DOI: 10.1523/JNEUROSCI.4720-06.2007
Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator
Abstract
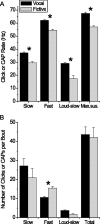
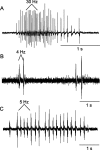
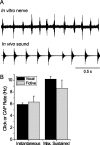
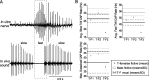
Male and female African clawed frogs (Xenopus laevis) produce rhythmic, sexually distinct vocalizations as part of courtship and mating. We found that Xenopus vocal behavior is governed by a sexually dimorphic central pattern generator (CPG) and that fictive vocalizations can be elicited from an in vitro brain preparation by application of serotonin or by electrical stimulation of a premotor nucleus. Male brains produced fictive vocal patterns representing two calls commonly produced by males in vivo (advertisement and amplectant call), as well as one call pattern (release call) that is common for juvenile males and females in vivo but rare for adult males. Female brains also produced fictive release call. The production of male calls is androgen dependent in Xenopus; to test the effects of androgens on the CPG, we examined fictive calling in the brains of testosterone-treated females. Both fictive male advertisement call and release call were produced. This suggests that all Xenopus possess a sexually undifferentiated pattern generator for release call. Androgen exposure leads to a gain-of-function, allowing the production of male-specific call types without prohibiting the production of the undifferentiated call pattern. We also demonstrate that the CPG is located in the brainstem and seems to rely on the same nuclei in both males and females. Finally, we identified endogenous serotonergic inputs to both the premotor and motor nuclei in the brainstem that may regulate vocal activity in vivo.
Figures



Similar articles
-
NMDAR-dependent control of call duration in Xenopus laevis.J Neurophysiol. 2010 Jun;103(6):3501-15. doi: 10.1152/jn.00155.2010. Epub 2010 Apr 14. J Neurophysiol. 2010. PMID: 20393064 Free PMC article.
-
Rhythm generation, coordination, and initiation in the vocal pathways of male African clawed frogs.J Neurophysiol. 2017 Jan 1;117(1):178-194. doi: 10.1152/jn.00628.2016. Epub 2016 Oct 19. J Neurophysiol. 2017. PMID: 27760822 Free PMC article.
-
Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female african clawed frogs (Xenopus laevis).J Neurosci. 2000 Feb 15;20(4):1559-67. doi: 10.1523/JNEUROSCI.20-04-01559.2000. J Neurosci. 2000. PMID: 10662845 Free PMC article.
-
Neuroeffectors for vocalization in Xenopus laevis: hormonal regulation of sexual dimorphism.J Neurobiol. 1986 May;17(3):231-48. doi: 10.1002/neu.480170307. J Neurobiol. 1986. PMID: 3519865 Review.
-
Generation, Coordination, and Evolution of Neural Circuits for Vocal Communication.J Neurosci. 2020 Jan 2;40(1):22-36. doi: 10.1523/JNEUROSCI.0736-19.2019. J Neurosci. 2020. PMID: 31896561 Free PMC article. Review.
Cited by
-
Distinct neural and neuromuscular strategies underlie independent evolution of simplified advertisement calls.Proc Biol Sci. 2013 Feb 13;280(1756):20122639. doi: 10.1098/rspb.2012.2639. Print 2013 Apr 7. Proc Biol Sci. 2013. PMID: 23407829 Free PMC article.
-
Oviposition-like central pattern generators in pregenital segments of male and female grasshoppers.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018 Apr;204(4):419-433. doi: 10.1007/s00359-018-1249-1. Epub 2018 Feb 8. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018. PMID: 29423751
-
Vocal and Electric Fish: Revisiting a Comparison of Two Teleost Models in the Neuroethology of Social Behavior.Front Neural Circuits. 2021 Aug 19;15:713105. doi: 10.3389/fncir.2021.713105. eCollection 2021. Front Neural Circuits. 2021. PMID: 34489647 Free PMC article. Review.
-
Probing forebrain to hindbrain circuit functions in Xenopus.Genesis. 2017 Jan;55(1-2):10.1002/dvg.22999. doi: 10.1002/dvg.22999. Genesis. 2017. PMID: 28095617 Free PMC article. Review.
-
Coding rate and duration of vocalizations of the frog, Xenopus laevis.J Neurosci. 2012 Aug 29;32(35):12102-14. doi: 10.1523/JNEUROSCI.2450-12.2012. J Neurosci. 2012. PMID: 22933794 Free PMC article.
References
-
- Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. - PubMed
-
- Birinyi A, Antal M, Wolf E, Szekely G. The extent of the dendritic tree and the number of synapses in the frog motoneuron. Eur J Neurosci. 1992;4:1003–1012. - PubMed
-
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-d-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
