Hypo-osmolar stress induces p75NTR expression by activating Sp1-dependent transcription
- PMID: 17287525
- PMCID: PMC6673569
- DOI: 10.1523/JNEUROSCI.4806-06.2007
Hypo-osmolar stress induces p75NTR expression by activating Sp1-dependent transcription
Abstract
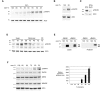
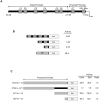
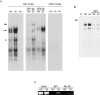
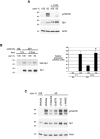
Injury-induced expression of the p75 neurotrophin receptor (p75NTR) in the CNS facilitates neuronal apoptosis and prevents neuronal regrowth, but the mechanisms regulating p75NTR expression are poorly characterized. In this study, we showed that hypo-osmolarity induces p75NTR expression in primary neurons, and, using a comparative genomics approach, we identified conserved elements in the 25 kb upstream sequences of the rat, mouse, and human p75NTR genes. We found that only one of these, a proximal region rich in Sp1 sites, responds to changes in hypo-osmolarity. We then showed that Sp1 DNA binding activity is increased in cells exposed to hypo-osmolarity, established that hypo-osmolarity enhanced Sp1 binding to the endogenous p75NTR promoter, and showed that Sp1 is required for p75NTR expression induced by hypo-osmolarity. We examined how Sp1 is regulated to effect these changes and established that Sp1 turnover is strongly inhibited by hypo-osmolarity. We propose that stress-induced Sp1 accumulation that results from reductions in Sp1 turnover rate contributes to injury-induced gene expression.
Figures



Similar articles
-
Stress-induced expression of the p75 neurotrophin receptor is regulated by O-GlcNAcylation of the Sp1 transcription factor.J Neurochem. 2011 Feb;116(3):396-405. doi: 10.1111/j.1471-4159.2010.07120.x. Epub 2010 Dec 13. J Neurochem. 2011. PMID: 21105874
-
Ethanol induces cell cycle arrest and triggers apoptosis via Sp1-dependent p75NTR expression in human neuroblastoma cells.Cell Biol Toxicol. 2013 Oct;29(5):365-80. doi: 10.1007/s10565-013-9260-3. Epub 2013 Sep 12. Cell Biol Toxicol. 2013. PMID: 24026251
-
Sp1 and Sp3 transcription factors mediate trichostatin A-induced and basal expression of extracellular superoxide dismutase.Free Radic Biol Med. 2004 Oct 15;37(8):1256-71. doi: 10.1016/j.freeradbiomed.2004.06.022. Free Radic Biol Med. 2004. PMID: 15451065
-
Molecular characterization of the constitutive expression of the plasma platelet-activating factor acetylhydrolase gene in macrophages.Biochem J. 2003 Oct 15;375(Pt 2):351-63. doi: 10.1042/BJ20030636. Biochem J. 2003. PMID: 12854969 Free PMC article.
-
Structure of the human 5-HT7 receptor gene and characterization of its promoter region.Gene. 2007 Apr 15;391(1-2):252-63. doi: 10.1016/j.gene.2007.01.003. Epub 2007 Jan 26. Gene. 2007. PMID: 17321075
Cited by
-
The loop diuretic bumetanide blocks posttraumatic p75NTR upregulation and rescues injured neurons.J Neurosci. 2012 Feb 1;32(5):1757-70. doi: 10.1523/JNEUROSCI.3282-11.2012. J Neurosci. 2012. PMID: 22302815 Free PMC article.
-
Proneurotrophins, seizures, and neuronal apoptosis.Neuroscientist. 2010 Jun;16(3):244-52. doi: 10.1177/1073858409349903. Epub 2010 Apr 1. Neuroscientist. 2010. PMID: 20360602 Free PMC article. Review.
-
Imbalance of p75(NTR)/TrkB protein expression in Huntington's disease: implication for neuroprotective therapies.Cell Death Dis. 2013 Apr 18;4(4):e595. doi: 10.1038/cddis.2013.116. Cell Death Dis. 2013. PMID: 23598407 Free PMC article.
-
Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4.J Neurosci. 2011 Apr 27;31(17):6392-7. doi: 10.1523/JNEUROSCI.6249-10.2011. J Neurosci. 2011. PMID: 21525279 Free PMC article.
-
Implication of the neurotrophin receptor p75NTR in vascular diseases: beyond the eye.Expert Rev Ophthalmol. 2017;12(2):149-158. doi: 10.1080/17469899.2017.1269602. Epub 2016 Dec 21. Expert Rev Ophthalmol. 2017. PMID: 28979360 Free PMC article.
References
-
- Andsberg G, Kokaia Z, Lindvall O. Upregulation of p75 neurotrophin receptor after stroke in mice does not contribute to differential vulnerability of striatal neurons. Exp Neurol. 2001;169:351–363. - PubMed
-
- Birnbaum MJ, van Wijnen AJ, Odgren PR, Last TJ, Suske G, Stein GS, Stein JL. Sp1 trans-activation of cell cycle regulated promoters is selectively repressed by Sp3. Biochemistry. 1995;34:16503–16508. - PubMed
-
- Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
