Valproic acid affects membrane trafficking and cell-wall integrity in fission yeast
- PMID: 17287531
- PMCID: PMC1855103
- DOI: 10.1534/genetics.107.070946
Valproic acid affects membrane trafficking and cell-wall integrity in fission yeast
Abstract
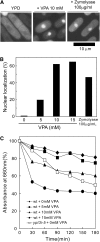
Valproic acid (VPA) is widely used to treat epilepsy and manic-depressive illness. Although VPA has been reported to exert a variety of biochemical effects, the exact mechanisms underlying its therapeutic effects remain elusive. To gain further insights into the molecular mechanisms of VPA action, a genetic screen for fission yeast mutants that show hypersensitivity to VPA was performed. One of the genes that we identified was vps45+, which encodes a member of the Sec1/Munc18 family that is implicated in membrane trafficking. Notably, several mutations affecting membrane trafficking also resulted in hypersensitivity to VPA. These include ypt3+ and ryh1+, both encoding a Rab family protein, and apm1+, encoding the mu1 subunit of the adaptor protein complex AP-1. More importantly, VPA caused vacuolar fragmentation and inhibited the glycosylation and the secretion of acid phosphatase in wild-type cells, suggesting that VPA affects membrane trafficking. Interestingly, the cell-wall-damaging agents such as micafungin or the inhibition of calcineurin dramatically enhanced the sensitivity of wild-type cells to VPA. Consistently, VPA treatment of wild-type cells enhanced their sensitivity to the cell-wall-digesting enzymes. Altogether, our results suggest that VPA affects membrane trafficking, which leads to the enhanced sensitivity to cell-wall damage in fission yeast.
Figures

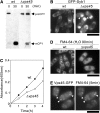
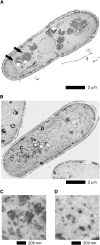

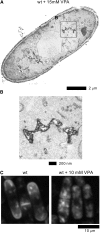

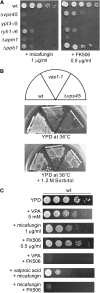
Similar articles
-
Isolation of a fission yeast mutant that is sensitive to valproic acid and defective in the gene encoding Ric1, a putative component of Ypt/Rab-specific GEF for Ryh1 GTPase.Mol Genet Genomics. 2010 Sep;284(3):161-71. doi: 10.1007/s00438-010-0550-7. Epub 2010 Jul 10. Mol Genet Genomics. 2010. PMID: 20623139
-
Genome-wide screening for genes associated with valproic acid sensitivity in fission yeast.PLoS One. 2013 Jul 5;8(7):e68738. doi: 10.1371/journal.pone.0068738. Print 2013. PLoS One. 2013. PMID: 23861937 Free PMC article.
-
Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast.Mol Biol Cell. 2008 Apr;19(4):1295-303. doi: 10.1091/mbc.e07-08-0805. Epub 2008 Jan 16. Mol Biol Cell. 2008. PMID: 18199682 Free PMC article.
-
Imp2, the PSTPIP homolog in fission yeast, affects sensitivity to the immunosuppressant FK506 and membrane trafficking in fission yeast.Biochem Biophys Res Commun. 2015 Feb 13;457(3):273-9. doi: 10.1016/j.bbrc.2014.12.100. Epub 2015 Jan 9. Biochem Biophys Res Commun. 2015. PMID: 25580011
-
Vesicle-mediated protein transport pathways to the vacuole in Schizosaccharomyces pombe.Cell Struct Funct. 2003 Oct;28(5):399-417. doi: 10.1247/csf.28.399. Cell Struct Funct. 2003. PMID: 14745133 Review.
Cited by
-
Isolation of a fission yeast mutant that is sensitive to valproic acid and defective in the gene encoding Ric1, a putative component of Ypt/Rab-specific GEF for Ryh1 GTPase.Mol Genet Genomics. 2010 Sep;284(3):161-71. doi: 10.1007/s00438-010-0550-7. Epub 2010 Jul 10. Mol Genet Genomics. 2010. PMID: 20623139
-
Phosphorylation of Calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus.PLoS Pathog. 2013;9(8):e1003564. doi: 10.1371/journal.ppat.1003564. Epub 2013 Aug 22. PLoS Pathog. 2013. PMID: 23990785 Free PMC article.
-
NADPH-Cytochrome P450 Reductase Ccr1 Is a Target of Tamoxifen and Participates in Its Antifungal Activity via Regulating Cell Wall Integrity in Fission Yeast.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00079-20. doi: 10.1128/AAC.00079-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571823 Free PMC article.
-
A genome-wide screening of potential target genes to enhance the antifungal activity of micafungin in Schizosaccharomyces pombe.PLoS One. 2013 May 30;8(5):e65904. doi: 10.1371/journal.pone.0065904. Print 2013. PLoS One. 2013. PMID: 23738021 Free PMC article.
-
Combined Transcriptomics and Chemical-Genetics Reveal Molecular Mode of Action of Valproic acid, an Anticancer Molecule using Budding Yeast Model.Sci Rep. 2016 Oct 13;6:35322. doi: 10.1038/srep35322. Sci Rep. 2016. PMID: 27734932 Free PMC article.
References
-
- Babst, M., G. Odorizzi, E. J. Estepa and S. D. Emr, 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248–258. - PubMed
-
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136. - PubMed
-
- Bates, S., D. M. MacCallum, G. Bertram, C. A. Munro, H. B. Hughes et al., 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280: 23408–23415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

