Products from enzyme-catalyzed oxidations of norcarenes
- PMID: 17288367
- PMCID: PMC2497458
- DOI: 10.1021/jo061865j
Products from enzyme-catalyzed oxidations of norcarenes
Abstract
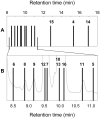
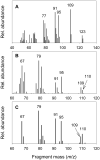
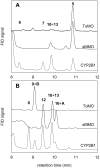
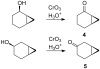
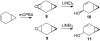
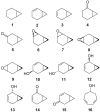
Recent studies revealed that norcarane (bicyclo[4.1.0]heptane) is oxidized to 2-norcarene (bicyclo[4.1.0]-hept-2-ene) and 3-norcarene (bicyclo[4.1.0]hept-3-ene) by iron-containing enzymes and that secondary oxidation products from the norcarenes complicate mechanistic probe studies employing norcarane as the substrate (Newcomb, M.; Chandrasena, R. E. P.; Lansakara-P., D. S. P.; Kim, H.-Y.; Lippard, S. J.; Beauvais, L. G.; Murray, L. J.; Izzo, V.; Hollenberg, P. F.; Coon, M. J. J. Org. Chem. 2007, 72, 1121-1127). In the present work, the product profiles from the oxidations of 2-norcarene and 3-norcarene by several enzymes were determined. Most of the products were identified by GC and GC-mass spectral comparison to authentic samples produced independently; in some cases, stereochemical assignments were made or confirmed by 2D NMR analysis of the products. The enzymes studied in this work were four cytochrome P450 enzymes, CYP2B1, CYPDelta2E1, CYPDelta2E1 T303A, and CYPDelta2B4, and three diiron-containing enzymes, soluble methane monooxygenase (sMMO) from Methylococcus capsulatus (Bath), toluene monooxygenase (ToMO) from Pseudomonas stutzeri OX1, and phenol hydroxylase (PH) from Pseudomonas stutzeri OX1. The oxidation products from the norcarenes identified in this work are 2-norcaranone, 3-norcaranone, syn- and anti-2-norcarene oxide, syn- and anti-3-norcarene oxide, syn- and anti-4-hydroxy-2-norcarene, syn- and anti-2-hydroxy-3-norcarene, 2-oxo-3-norcarene, 4-oxo-2-norcarene, and cyclohepta-3,5-dienol. Two additional, unidentified oxidation products were observed in low yields in the oxidations. In matched oxidations, 3-norcarene was a better substrate than 2-norcarene in terms of turnover by factors of 1.5-15 for the enzymes studied here. The oxidation products found in enzyme-catalyzed oxidations of the norcarenes are useful for understanding the complex product mixtures obtained in norcarane oxidations.
Figures


References
-
- Newcomb M, Shen RN, Lu Y, Coon MJ, Hollenberg PF, Kopp DA, Lippard SJ. J Am Chem Soc. 2002;124:6879–6886. - PubMed
-
- Newcomb M, Shen RN, Lu Y, Coon MJ, Hollenberg PF, Kopp DA, Lippard SJ. J Am Chem Soc. 2006;128:1394. - PubMed
-
- Newcomb M, Chandrasena REP, Lansakara-P DSP, Kim HY, Lippard SJ, Beauvais LG, Murray LJ, Izzo V, Hollenberg PF, Coon MJ. Accompanying paper.
-
- Chan JHH, Rickborn B. J Am Chem Soc. 1968;90:6406–6411.
-
- Lukin KA, Zefirov NS. Zhur Organich Khim. 1988;24:1648–1652.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

