Conformational rigidity of silicon-stereogenic silanes in asymmetric catalysis: A comparative study
- PMID: 17288605
- PMCID: PMC1810299
- DOI: 10.1186/1860-5397-3-9
Conformational rigidity of silicon-stereogenic silanes in asymmetric catalysis: A comparative study
Abstract
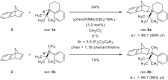
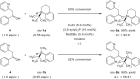
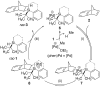
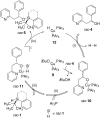
In recent years, cyclic silicon-stereogenic silanes were successfully employed as stereoinducers in transition metal-catalyzed asymmetric transformations as exemplified by (1) the hydrosilylation of alkenes constituting a chirality transfer from silicon to carbon and (2) the kinetic resolution of racemic mixtures of alcohols by dehydrogenative silicon-oxygen coupling. In this investigation, a cyclic and a structurally related acyclic silane with silicon-centered chirality were compared using the above-mentioned model reactions. The stereochemical outcome of these pairs of reactions was correlated with and rationalized by the current mechanistic pictures. An acyclic silicon-stereogenic silane is also capable of inducing excellent chirality transfer (ct) in a palladium-catalyzed intermolecular carbon-silicon bond formation yet silicon incorporated into a cyclic framework is required in the copper-catalyzed silicon-oxygen bond forming reaction.
Figures
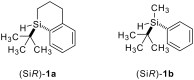
References
-
- Maryanoff C A, Maryanoff B E. In: Synthesis and Utilization of Compounds with Chiral Silicon Centers. Asymmetric Synthesis. Morrison J D, Scott J W, editors. Vol. 4. Orlando: Academic Press; 1984. pp. 355–374.
-
- Sommer L H. Stereochemistry, Mechanism and Silicon. New York: McGraw-Hill; 1965.
-
- Sommer L H. Intra-Sci Chem Rep. 1973;7:1–44.
-
- Corriu R J P, Guérin C, Moreau J J E. In: Stereochemistry at Silicon. Topics in Stereochemistry. Eliel E L, editor. Vol. 15. New York: Wiley; 1984. pp. 43–198.
-
- Bienz S. Chimia. 1997;51:133–139.
LinkOut - more resources
Full Text Sources
