The Krüppel-like zinc finger protein Glis2 functions as a negative modulator of the Wnt/beta-catenin signaling pathway
- PMID: 17289029
- PMCID: PMC1838965
- DOI: 10.1016/j.febslet.2007.01.058
The Krüppel-like zinc finger protein Glis2 functions as a negative modulator of the Wnt/beta-catenin signaling pathway
Abstract
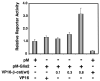
To gain insight into the mechanism by which Gli-similar 2 (Glis2) regulates transcription, we performed yeast-two hybrid cDNA library screening using Glis2 as bait. This screening identified beta-catenin as a potential Glis2-interacting protein. Mammalian two-hybrid, co-immunoprecipitation, and GST-pulldown analyses supported the interaction between Glis2 and beta-catenin. Pulldown analyses with several Glis2 deletion mutants indicated that the 1st zinc finger motif of Glis2 is critical for its interaction with beta-catenin, while the armadillo repeats of beta-catenin are important in its interaction with Glis2. Reporter analyses showed that Glis2 represses T-cell factor (TCF)-mediated transcriptional activation. In addition, Glis2 represses the expression of the TCF target gene cyclin D1. Our results indicate that Glis2 interacts with beta-catenin and suggest that Glis2 functions as a negative modulator of beta-catenin/TCF-mediated transcription.
Figures

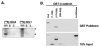
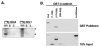




References
-
- Kim YS, Lewandoski M, Perantoni AO, Kurebayashi S, Nakanishi G, Jetten AM. Identification of Glis1, a novel Gli-related, Kruppel-like zinc finger protein containing transactivation and repressor functions. J. Biol. Chem. 2002;277:30901–30913. - PubMed
-
- Lamar E, Kintner C, Goulding M. Identification of NKL, a novel Gli-Kruppel zinc-finger protein that promotes neuronal differentiation. Development. 2001;128:1335–1346. - PubMed
-
- Zhang F, Nakanishi G, Kurebayashi S, Yoshino K, Perantoni A, Kim YS, Jetten AM. Characterization of Glis2, a novel gene encoding a Gli-related, Kruppel-like transcription factor with transactivation and repressor functions. Roles in kidney development and neurogenesis. J. Biol. Chem. 2002;277:10139–10149. - PubMed
-
- Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

