Ribosomal protein L20 controls expression of the Bacillus subtilis infC operon via a transcription attenuation mechanism
- PMID: 17289755
- PMCID: PMC1865079
- DOI: 10.1093/nar/gkm011
Ribosomal protein L20 controls expression of the Bacillus subtilis infC operon via a transcription attenuation mechanism
Abstract
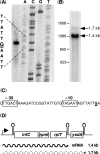
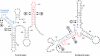
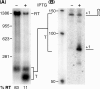
In contrast to Escherichia coli no molecular mechanism controlling the biosynthesis of ribosomal proteins has been elucidated in Gram-positive organisms. Here we show that the expression of the Bacillus subtilis infC-rpmI-rplT operon encoding translation factor IF3 and the ribosomal proteins L35 and L20 is autoregulated by a complex transcription attenuation mechanism. It implicates a 200-bp leader region upstream of infC which contains two conserved regulatory elements, one of which can act as a transcription terminator. Using in vitro and in vivo approaches we show that expression of the operon is regulated at the level of transcription elongation by a change in the structure of the leader mRNA which depends upon the presence of ribosomal protein L20. L20 binds to a phylogenetically conserved domain and provokes premature transcription termination at the leader terminator. Footprint and toeprint experiments support a regulatory model involving molecular mimicry between the L20-binding sites on 23S rRNA and the mRNA. Our data suggest that Nomura's model of ribosomal protein biosynthesis based on autogenous control and molecular mimicry is also valid in Gram-positive organisms.
Figures


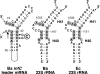


References
-
- Zengel JM, Lindahl L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog. Nucleic Acid Res. Mol. Biol. 1994;47:331–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

