New thrombopoietic growth factors
- PMID: 17289815
- PMCID: PMC1885525
- DOI: 10.1182/blood-2006-10-019315
New thrombopoietic growth factors
Abstract
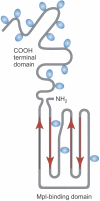
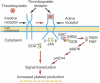
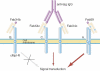
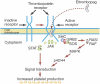
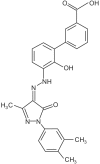
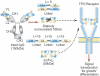
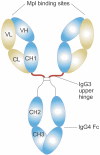
Although development of first-generation thrombopoietic growth factors (recombinant human thrombopoietin [TPO] and pegylated recombinant human megakaryocyte growth and development factor [PEG-rHuMGDF]) was stopped due to development of antibodies to PEG-rHuMGDF, nonimmunogenic second-generation thrombopoietic growth factors with unique pharmacologic properties have been developed. TPO peptide mimetics contain TPO receptor-activating peptides inserted into complementarity-determining regions of Fab (Fab 59), attached to the IgG Fc region (AMG 531), or pegylated (Peg-TPOmp). Orally available, TPO nonpeptide mimetics (eltrombopag, AKR-501) bind and activate the TPO receptor by a mechanism different from TPO and may have an additive effect to TPO. TPO agonist antibodies are monoclonal antibodies activating the TPO receptor but modified in size [TPO minibodies; ie, VB22B sc(Fv)(2)] or immunoglobuln type (domain subclass-converted TPO agonist antibodies; ie, MA01G4G344). All second-generation thrombopoietic growth factors stimulate growth of TPO-dependent cell lines via JAK2/STAT signaling pathways and increase platelet counts in animals. When tested in healthy humans, TPO peptide and nonpeptide mimetics produced a dose-dependent rise in platelet count. AMG 531 and eltrombopag markedly increase platelet counts in patients with immune thrombocytopenic purpura, without significant adverse effects. One or more second-generation thrombopoietic growth factors should soon be clinically available for treating thrombocytopenic disorders.
Figures
References
-
- Bartley TD, Bogenberger J, Hunt P, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77:1117–1124. - PubMed
-
- Lok S, Kaushansky K, Holly RD, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. - PubMed
-
- Kato T, Ogami K, Shimada Y, et al. Purification and characterization of thrombopoietin. J Biochem. 1995;118:229–236. - PubMed
-
- de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

