Role of microsomal retinol/sterol dehydrogenase-like short-chain dehydrogenases/reductases in the oxidation and epimerization of 3alpha-hydroxysteroids in human tissues
- PMID: 17289849
- PMCID: PMC2571913
- DOI: 10.1210/en.2006-1491
Role of microsomal retinol/sterol dehydrogenase-like short-chain dehydrogenases/reductases in the oxidation and epimerization of 3alpha-hydroxysteroids in human tissues
Abstract
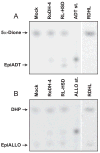
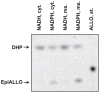
Allopregnanolone (ALLO) and androsterone (ADT) are naturally occurring 3alpha-hydroxysteroids that act as positive allosteric regulators of gamma-aminobutyric acid type A receptors. In addition, ADT activates nuclear farnesoid X receptor and ALLO activates pregnane X receptor. At least with respect to gamma-aminobutyric acid type A receptors, the biological activity of ALLO and ADT depends on the 3alpha-hydroxyl group and is lost upon its conversion to either 3-ketosteroid or 3beta-hydroxyl epimer. Such strict structure-activity relationships suggest that the oxidation or epimerization of 3alpha-hydroxysteroids may serve as physiologically relevant mechanisms for the control of the local concentrations of bioactive 3alpha-hydroxysteroids. The exact enzymes responsible for the oxidation and epimerization of 3alpha-hydroxysteroids in vivo have not yet been identified, but our previous studies showed that microsomal nicotinamide adenine dinucleotide-dependent short-chain dehydrogenases/reductases (SDRs) with dual retinol/sterol dehydrogenase substrate specificity (RoDH-like group of SDRs) can oxidize and epimerize 3alpha-hydroxysteroids in vitro. Here, we present the first evidence that microsomal nicotinamide adenine dinucleotide-dependent 3alpha-hydroxysteroid dehydrogenase/epimerase activities are widely distributed in human tissues with the highest activity levels found in liver and testis and lower levels in lung, spleen, brain, kidney, and ovary. We demonstrate that RoDH-like SDRs contribute to the oxidation and epimerization of ALLO and ADT in living cells, and show that RoDH enzymes are expressed in tissues that have microsomal 3alpha-hydroxysteroid dehydrogenase/epimerase activities. Together, these results provide further support for the role of RoDH-like SDRs in human metabolism of 3alpha-hydroxysteroids and offer a new insight into the enzymology of ALLO and ADT inactivation.
Figures





References
-
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. - PubMed
-
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. - PubMed
-
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. - PubMed
-
- Baulieu EE, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum Reprod. 2000;15:1–13. - PubMed
-
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. - PubMed

