Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development
- PMID: 17289920
- PMCID: PMC1785125
- DOI: 10.1101/gad.398207
Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development
Abstract
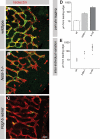
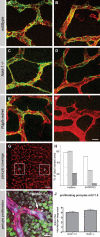
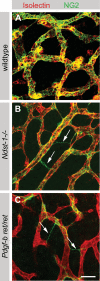
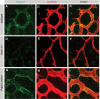
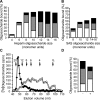
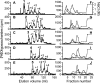
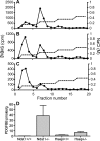
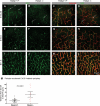
During vascular development, endothelial platelet-derived growth factor B (PDGF-B) is critical for pericyte recruitment. Deletion of the conserved C-terminal heparin-binding motif impairs PDGF-BB retention and pericyte recruitment in vivo, suggesting a potential role for heparan sulfate (HS) in PDGF-BB function during vascular development. We studied the participation of HS chains in pericyte recruitment using two mouse models with altered HS biosynthesis. Reduction of N-sulfation due to deficiency in N-deacetylase/N-sulfotransferase-1 attenuated PDGF-BB binding in vitro, and led to pericyte detachment and delayed pericyte migration in vivo. Reduced N-sulfation also impaired PDGF-BB signaling and directed cell migration, but not proliferation. In contrast, HS from glucuronyl C5-epimerase mutants, which is extensively N- and 6-O-sulfated, but lacks 2-O-sulfated L-iduronic acid residues, retained PDGF-BB in vitro, and pericyte recruitment in vivo was only transiently delayed. These observations were supported by in vitro characterization of the structural features in HS important for PDGF-BB binding. We conclude that pericyte recruitment requires HS with sufficiently extended and appropriately spaced N-sulfated domains to retain PDGF-BB and activate PDGF receptor beta (PDGFRbeta) signaling, whereas the detailed sequence of monosaccharide and sulfate residues does not appear to be important for this interaction.
Figures
References
-
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. - PubMed
-
- Bjarnegard M., Enge M., Norlin J., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Enge M., Norlin J., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Norlin J., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Abramsson A., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Takemoto M., Gustafsson E., Fassler R., Betsholtz C., Gustafsson E., Fassler R., Betsholtz C., Fassler R., Betsholtz C., Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. - PubMed
-
- Couchman J.R., Woods A., Woods A. Syndecan-4 and integrins: Combinatorial signaling in cell adhesion. J. Cell Sci. 1999;112:3415–3420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
