Cdk phosphorylation of the Ste11 transcription factor constrains differentiation-specific transcription to G1
- PMID: 17289922
- PMCID: PMC1785116
- DOI: 10.1101/gad.407107
Cdk phosphorylation of the Ste11 transcription factor constrains differentiation-specific transcription to G1
Abstract
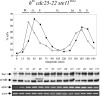
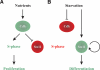
Eukaryotic cells normally differentiate from G(1); here we investigate the mechanism preventing expression of differentiation-specific genes outside G(1). In fission yeast, induction of the transcription factor Ste11 triggers sexual differentiation. We find that Ste11 is only active in G(1) when Cdk activity is low. In the remaining part of the cell cycle, Ste11 becomes Cdk-phosphorylated at Thr 82 (T82), which inhibits its DNA-binding activity. Since the ste11 gene is autoregulated and the Ste11 protein is highly unstable, this Cdk switch rapidly extinguishes Ste11 activity when cells enter S phase. When we mutated T82 to aspartic acid, mimicking constant phosphorylation, cells no longer underwent differentiation. Conversely, changing T82 to alanine rendered Ste11-controlled transcription constitutive through the cell cycle, and allowed mating from S phase with increased frequency. Thus, Cdk phosphorylation mediates periodic expression of Ste11 and its target genes, and we suggest this to be part of the mechanism restricting differentiation to G(1).
Figures



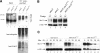

Similar articles
-
Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast.EMBO J. 2008 Jan 9;27(1):132-42. doi: 10.1038/sj.emboj.7601949. Epub 2007 Dec 6. EMBO J. 2008. PMID: 18059475 Free PMC article.
-
Fission yeast Fizzy-related protein srw1p is a G(1)-specific promoter of mitotic cyclin B degradation.EMBO J. 2000 Aug 1;19(15):3968-77. doi: 10.1093/emboj/19.15.3968. EMBO J. 2000. PMID: 10921878 Free PMC article.
-
G(1)/S CDK is inhibited to restrain mitotic onset when DNA replication is blocked in fission yeast.EMBO J. 2002 Jul 1;21(13):3370-6. doi: 10.1093/emboj/cdf346. EMBO J. 2002. PMID: 12093738 Free PMC article.
-
Down-regulation of Cdk1 activity in G1 coordinates the G1/S gene expression programme with genome replication.Curr Genet. 2019 Jun;65(3):685-690. doi: 10.1007/s00294-018-00926-y. Epub 2019 Jan 24. Curr Genet. 2019. PMID: 30680437 Review.
-
Modelling the CDK-dependent transcription cycle in fission yeast.Biochem Soc Trans. 2013 Dec;41(6):1660-5. doi: 10.1042/BST20130238. Biochem Soc Trans. 2013. PMID: 24256271 Review.
Cited by
-
Computational modelling of meiotic entry and commitment.Sci Rep. 2018 Jan 9;8(1):180. doi: 10.1038/s41598-017-17478-9. Sci Rep. 2018. PMID: 29317645 Free PMC article.
-
Neurospora crassa fmf-1 encodes the homologue of the Schizosaccharomyces pombe Ste11p regulator of sexual development.J Genet. 2009 Apr;88(1):33-9. doi: 10.1007/s12041-009-0005-2. J Genet. 2009. PMID: 19417542
-
Sporulation: A response to starvation in the fission yeast Schizosaccharomyces pombe.Microbiologyopen. 2022 Jun;11(3):e1303. doi: 10.1002/mbo3.1303. Microbiologyopen. 2022. PMID: 35765188 Free PMC article. Review.
-
The S. pombe SAGA complex controls the switch from proliferation to sexual differentiation through the opposing roles of its subunits Gcn5 and Spt8.Genes Dev. 2008 Nov 15;22(22):3184-95. doi: 10.1101/gad.1719908. Genes Dev. 2008. PMID: 19056896 Free PMC article.
-
Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast.EMBO J. 2008 Jan 9;27(1):132-42. doi: 10.1038/sj.emboj.7601949. Epub 2007 Dec 6. EMBO J. 2008. PMID: 18059475 Free PMC article.
References
-
- Booher R.N., Alfa C.E., Hyams J.S., Beach D.H., Alfa C.E., Hyams J.S., Beach D.H., Hyams J.S., Beach D.H., Beach D.H. The fission yeast cdc2/cdc13/suc1 protein kinase: Regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. - PubMed
-
- Costanzo M., Nishikawa J.L., Tang X., Millman J.S., Schub O., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Nishikawa J.L., Tang X., Millman J.S., Schub O., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Tang X., Millman J.S., Schub O., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Millman J.S., Schub O., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Schub O., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Breitkreuz K., Dewar D., Rupes I., Andrews B., Tyers M., Dewar D., Rupes I., Andrews B., Tyers M., Rupes I., Andrews B., Tyers M., Andrews B., Tyers M., Tyers M. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell. 2004;117:899–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
