Opposed growth factor signals control protein degradation in muscles of Caenorhabditis elegans
- PMID: 17290229
- PMCID: PMC1852841
- DOI: 10.1038/sj.emboj.7601540
Opposed growth factor signals control protein degradation in muscles of Caenorhabditis elegans
Abstract
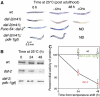
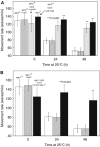
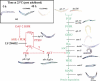
In addition to contractile function, muscle provides a metabolic buffer by degrading protein in times of organismal need. Protein is also degraded during adaptive muscle remodeling upon exercise, but extreme degradation in diverse clinical conditions can compromise function(s) and threaten life. Here, we show how two independent signals interact to control protein degradation. In striated muscles of Caenorhabditis elegans, reduction of insulin-like signaling via DAF-2 insulin/IGF receptor or its intramuscular effector PtdIns-3-kinase (PI3K) causes unexpected activation of MAP kinase (MAPK), consequent activation of pre-existing systems for protein degradation, and progressive impairment of mobility. Degradation is prevented by mutations that increase signal downstream of PI3K or by disruption of autocrine signal from fibroblast growth factor (FGF) via the FGF receptor and its effectors in the Ras-MAPK pathway. Thus, the activity of constitutive protein degradation systems in normal muscle is minimized by a balance between directly interacting signaling pathways, implying that physiological, pathological, or therapeutic alteration of this balance may contribute to muscle remodeling or wasting.
Figures




Similar articles
-
Signal-transduction networks and the regulation of muscle protein degradation.Int J Biochem Cell Biol. 2005 Oct;37(10):1997-2011. doi: 10.1016/j.biocel.2005.02.020. Epub 2005 Mar 14. Int J Biochem Cell Biol. 2005. PMID: 16125109 Review.
-
Activated EGL-15 FGF receptor promotes protein degradation in muscles of Caenorhabditis elegans.EMBO J. 2003 Oct 1;22(19):5058-67. doi: 10.1093/emboj/cdg472. EMBO J. 2003. PMID: 14517244 Free PMC article.
-
LET-60 RAS modulates effects of insulin/IGF-1 signaling on development and aging in Caenorhabditis elegans.Aging Cell. 2005 Oct;4(5):235-45. doi: 10.1111/j.1474-9726.2005.00166.x. Aging Cell. 2005. PMID: 16164423
-
Insulin-like signaling negatively regulates muscle arm extension through DAF-12 in Caenorhabditis elegans.Dev Biol. 2008 Jun 1;318(1):153-61. doi: 10.1016/j.ydbio.2008.03.019. Epub 2008 Mar 20. Dev Biol. 2008. PMID: 18436204
-
Skeletal muscle hypertrophy and atrophy signaling pathways.Int J Biochem Cell Biol. 2005 Oct;37(10):1974-84. doi: 10.1016/j.biocel.2005.04.018. Int J Biochem Cell Biol. 2005. PMID: 16087388 Review.
Cited by
-
Eldecalcitol prevents muscle loss by suppressing PI3K/AKT/FOXOs pathway in orchiectomized mice.Front Pharmacol. 2022 Oct 28;13:1018480. doi: 10.3389/fphar.2022.1018480. eCollection 2022. Front Pharmacol. 2022. PMID: 36386197 Free PMC article.
-
Greater loss of mitochondrial function with ageing is associated with earlier onset of sarcopenia in C. elegans.Aging (Albany NY). 2018 Nov 19;10(11):3382-3396. doi: 10.18632/aging.101654. Aging (Albany NY). 2018. PMID: 30455409 Free PMC article.
-
Knockdown of the C. elegans kinome identifies kinases required for normal protein homeostasis, mitochondrial network structure, and sarcomere structure in muscle.Cell Commun Signal. 2013 Sep 23;11:71. doi: 10.1186/1478-811X-11-71. Cell Commun Signal. 2013. PMID: 24060339 Free PMC article.
-
Methods to assess subcellular compartments of muscle in C. elegans.J Vis Exp. 2014 Nov 13;(93):e52043. doi: 10.3791/52043. J Vis Exp. 2014. PMID: 25489753 Free PMC article.
-
A role for autophagy in the extension of lifespan by dietary restriction in C. elegans.PLoS Genet. 2008 Feb;4(2):e24. doi: 10.1371/journal.pgen.0040024. PLoS Genet. 2008. PMID: 18282106 Free PMC article.
References
-
- Andreatta C, Nahreini P, Hovland B, Kumar J, Edwards-Prasad J, Prasad K (2001) Use of short-lived green fluorescent protein for the detection of proteasome inhibition. BioTechniques 30: 656–660 - PubMed
-
- Attaix D, Combaret L, Pouch MN, Taillandier D (2001) Regulation of proteolysis. Curr Opin Clin Nutr Metab Care 4: 45–49 - PubMed
-
- Avery AW, Figueroa C, Vojtek AB (2007) UNC-51-like kinase regulation of fibroblast growth factor receptor substrate 2/3. Cell Signal 19: 177–184 - PubMed
-
- Babar P, Adamson C, Walker GA, Walker DW, Lithgow GJ (1999) P13-kinase inhibition induces dauer formation, thermotolerance and longevity in C. elegans. Neurobiol Aging 20: 513–519 - PubMed
-
- Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292: 1552–1555 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

