Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism
- PMID: 17290304
- PMCID: PMC1784003
- DOI: 10.1172/JCI30142
Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism
Abstract
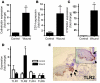
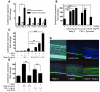
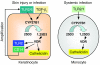
An essential element of the innate immune response to injury is the capacity to recognize microbial invasion and stimulate production of antimicrobial peptides. We investigated how this process is controlled in the epidermis. Keratinocytes surrounding a wound increased expression of the genes coding for the microbial pattern recognition receptors CD14 and TLR2, complementing an increase in cathelicidin antimicrobial peptide expression. These genes were induced by 1,25(OH)2 vitamin D3 (1,25D3; its active form), suggesting a role for vitamin D3 in this process. How 1,25D3 could participate in the injury response was explained by findings that the levels of CYP27B1, which converts 25OH vitamin D3 (25D3) to active 1,25D3, were increased in wounds and induced in keratinocytes in response to TGF-beta1. Blocking the vitamin D receptor, inhibiting CYP27B1, or limiting 25D3 availability prevented TGF-beta1 from inducing cathelicidin, CD14, or TLR2 in human keratinocytes, while CYP27B1-deficient mice failed to increase CD14 expression following wounding. The functional consequence of these observations was confirmed by demonstrating that 1,25D3 enabled keratinocytes to recognize microbial components through TLR2 and respond by cathelicidin production. Thus, we demonstrate what we believe to be a previously unexpected role for vitamin D3 in innate immunity, enabling keratinocytes to recognize and respond to microbes and to protect wounds against infection.
Figures
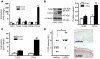
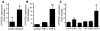
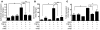
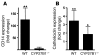
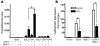
Similar articles
-
Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3.J Invest Dermatol. 2008 Apr;128(4):816-24. doi: 10.1038/sj.jid.5701102. Epub 2007 Oct 18. J Invest Dermatol. 2008. PMID: 17943182
-
Mycobacterium avium paratuberculosis Infection Suppresses Vitamin D Activation and Cathelicidin Production in Macrophages through Modulation of the TLR2-Dependent p38/MAPK-CYP27B1-VDR-CAMP Axis.Nutrients. 2024 Apr 30;16(9):1358. doi: 10.3390/nu16091358. Nutrients. 2024. PMID: 38732603 Free PMC article.
-
IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway.J Immunol. 2008 Nov 15;181(10):7115-20. doi: 10.4049/jimmunol.181.10.7115. J Immunol. 2008. PMID: 18981132 Free PMC article.
-
Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production.Inflamm Res. 2016 Jan;65(1):25-32. doi: 10.1007/s00011-015-0884-z. Epub 2015 Oct 3. Inflamm Res. 2016. PMID: 26433491 Review.
-
[New insights in the pathogenesis and treatment of rosacea].Duodecim. 2012;128(22):2327-35. Duodecim. 2012. PMID: 23342479 Review. Finnish.
Cited by
-
The dark side of daylight: photoaging and the tumor microenvironment in melanoma progression.J Clin Invest. 2021 Mar 15;131(6):e143763. doi: 10.1172/JCI143763. J Clin Invest. 2021. PMID: 33720046 Free PMC article. Review.
-
Cytochrome P450-mediated metabolism of vitamin D.J Lipid Res. 2014 Jan;55(1):13-31. doi: 10.1194/jlr.R031534. Epub 2013 Apr 6. J Lipid Res. 2014. PMID: 23564710 Free PMC article. Review.
-
Evaluation of Vitamin D Status in Newly Diagnosed Pemphigus Vulgaris Patients.Iran J Public Health. 2014 Nov;43(11):1544-9. Iran J Public Health. 2014. PMID: 26060722 Free PMC article.
-
How important is vitamin D in preventing infections?Osteoporos Int. 2013 May;24(5):1537-53. doi: 10.1007/s00198-012-2204-6. Epub 2012 Nov 17. Osteoporos Int. 2013. PMID: 23160915 Review.
-
New tricks for old dogs: countering antibiotic resistance in tuberculosis with host-directed therapeutics.Immunol Rev. 2015 Mar;264(1):344-62. doi: 10.1111/imr.12255. Immunol Rev. 2015. PMID: 25703571 Free PMC article. Review.
References
-
- Roth D.E., et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J. Infect. Dis. 2004;190:920–927. - PubMed
-
- Wayse V., Yousafzai A., Mogale K., Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur. J. Clin. Nutr. 2004;58:563–567. - PubMed
-
- Liu P.T., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. - PubMed
-
- Zasloff M. Fighting infections with vitamin D. Nat. Med. 2006;12:388–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

