Investigation of the reaction of myosmine with sodium nitrite in vitro and in rats
- PMID: 17291014
- PMCID: PMC2518846
- DOI: 10.1021/tx600328e
Investigation of the reaction of myosmine with sodium nitrite in vitro and in rats
Abstract
Previous studies have shown that the minor tobacco alkaloid myosmine (5) reacts with NaNO2 in the presence of acid to yield 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB, 8) via 4-(3-pyridyl)-4-oxobutanediazohydroxide (7). Intermediate 7 is also formed in the metabolism of the tobacco-specific nitrosamines N'-nitrosonornicotine (NNN, 1) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 2), resulting in pyridyloxobutylation of DNA and Hb. These pyridyloxobutyl adducts can be quantified by analyzing HPB released upon acid treatment of DNA or base treatment of Hb. Quantitation of HPB-releasing DNA and Hb adducts has been used to assess the metabolic activation of NNN and NNK in smokers and smokeless tobacco users. Because myosmine is found in the diet as well as in tobacco products, it has been suggested that nitrosation of myosmine could lead to the formation of HPB-releasing adducts in people not exposed to tobacco products. We investigated the nitrosation of myosmine in vitro and in vivo in rats. The reaction of myosmine with NaNO2 under acidic conditions produced HPB, as previously reported. A new product was identified as 3'-oximinomyosmine (11) based on its spectral properties. NNN was not detected. Groups of rats were treated with NNN, NNK, myosmine, NaNO2, or combinations of myosmine and NaNO2. HPB-releasing Hb and DNA adducts were clearly detected in the rats treated with NNN or NNK, but we found no evidence for production of these adducts from the combination of myosmine plus NaNO2. The results of this study do not support the hypothesis that exposure to dietary myosmine could lead to HPB-releasing DNA or Hb adducts in humans.
Figures



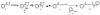
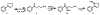
References
-
- Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev Cancer. 2003;3:733–744. - PubMed
-
- Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–294. - PubMed
-
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. - PubMed
-
- Jalas J, Hecht SS, Murphy SE. Cytochrome P450 2A enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific carcinogen. Chem Res Toxicol. 2005;18:95–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

