Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation
- PMID: 17291192
- PMCID: PMC1876384
- DOI: 10.1042/BJ20061709
Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation
Abstract
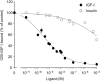
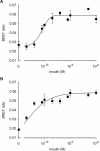
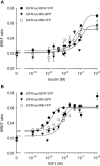
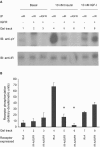
The IR (insulin receptor) and IGFR (type I insulin-like growth factor receptor) are found as homodimers, but the respective pro-receptors can also heterodimerize to form insulin-IGF hybrid receptors. There are conflicting data on the ligand affinity of hybrids, and especially on the influence of different IR isoforms. To investigate further the contribution of individual ligand binding epitopes to affinity and specificity in the IR/IGFR family, we generated hybrids incorporating both IR isoforms (A and B) and IR/IGFR domain-swap chimaeras, by ectopic co-expression of receptor constructs in Chinese hamster ovary cells, and studied ligand binding using both radioligand competition and bioluminescence resonance energy transfer assays. We found that IR-A-IGFR and IR-B-IGFR hybrids bound insulin with similar relatively low affinity, which was intermediate between that of homodimeric IR and homodimeric IGFR. However, both IR-A-IGFR and IR-B-IGFR hybrids bound IGF-I and IGF-II with high affinity, at a level comparable with homodimeric IGFR. Incorporation of a significant fraction of either IR-A or IR-B into hybrids resulted in abrogation of insulin- but not IGF-I-stimulated autophosphorylation. We conclude that the sequence of 12 amino acids encoded by exon 11 of the IR gene has little or no effect on ligand binding and activation of IR-IGFR hybrids, and that hybrid receptors bind IGFs but not insulin at physiological concentrations regardless of the IR isoform they contained. To reconstitute high affinity insulin binding within a hybrid receptor, chimaeras in which the IGFR L1 or L2 domains had been replaced by equivalent IR domains were co-expressed with full-length IR-A or IR-B. In the context of an IR-A-IGFR hybrid, replacement of IR residues 325-524 (containing the L2 domain and part of the first fibronectin domain) with the corresponding IGFR sequence increased the affinity for insulin by 20-fold. We conclude that the L2 and/or first fibronectin domains of IR contribute in trans with the L1 domain to create a high affinity insulin-binding site within a dimeric receptor.
Figures




Similar articles
-
Insulin/insulin-like growth factor-I hybrid receptors with high affinity for insulin are developmentally regulated during neurogenesis.Endocrinology. 1999 Jan;140(1):233-43. doi: 10.1210/endo.140.1.6393. Endocrinology. 1999. PMID: 9886830
-
Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting.Biochem J. 1997 Oct 1;327 ( Pt 1)(Pt 1):209-15. doi: 10.1042/bj3270209. Biochem J. 1997. PMID: 9355755 Free PMC article.
-
Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR.Mol Endocrinol. 2004 Oct;18(10):2502-12. doi: 10.1210/me.2004-0183. Epub 2004 Jun 17. Mol Endocrinol. 2004. PMID: 15205474
-
Structural insights into ligand-induced activation of the insulin receptor.Acta Physiol (Oxf). 2008 Jan;192(1):3-9. doi: 10.1111/j.1748-1716.2007.01781.x. Acta Physiol (Oxf). 2008. PMID: 18171424 Review.
-
Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors.Biochem Soc Trans. 2001 Aug;29(Pt 4):513-25. doi: 10.1042/bst0290513. Biochem Soc Trans. 2001. PMID: 11498020 Review.
Cited by
-
Harmonic oscillator model of the insulin and IGF1 receptors' allosteric binding and activation.Mol Syst Biol. 2009;5:243. doi: 10.1038/msb.2008.78. Epub 2009 Feb 17. Mol Syst Biol. 2009. PMID: 19225456 Free PMC article.
-
The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1).Front Neuroendocrinol. 2014 Oct;35(4):558-72. doi: 10.1016/j.yfrne.2014.05.007. Epub 2014 Jun 12. Front Neuroendocrinol. 2014. PMID: 24929098 Free PMC article. Review.
-
Insulin Receptor Isoforms in Physiology and Disease: An Updated View.Endocr Rev. 2017 Oct 1;38(5):379-431. doi: 10.1210/er.2017-00073. Endocr Rev. 2017. PMID: 28973479 Free PMC article. Review.
-
Heat shock response and insulin-associated neurodegeneration.Trends Pharmacol Sci. 2012 Mar;33(3):129-37. doi: 10.1016/j.tips.2011.11.001. Epub 2011 Dec 13. Trends Pharmacol Sci. 2012. PMID: 22172248 Free PMC article. Review.
-
Understanding the mechanism of insulin and insulin-like growth factor (IGF) receptor activation by IGF-II.PLoS One. 2011;6(11):e27488. doi: 10.1371/journal.pone.0027488. Epub 2011 Nov 28. PLoS One. 2011. PMID: 22140443 Free PMC article.
References
-
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E., et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. - PMC - PubMed
-
- Olson T. S., Bamberger M. J., Lane M. D. Post-translational changes in tertiary and quaternary structure of the insulin proreceptor: correlation with acquisition of function. J. Biol. Chem. 1988;263:7342–7351. - PubMed
-
- Moxham C. P., Duronio V., Jacobs S. Insulin-like growth factor I receptor β-subunit heterogeneity: evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J. Biol. Chem. 1989;264:13238–13244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

