Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels
- PMID: 17291197
- PMCID: PMC1868827
- DOI: 10.1042/BJ20061404
Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels
Abstract
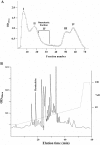
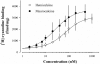
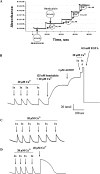
In the present work, we purified and characterized a novel toxin named hemicalcin from the venom of the Iranian chactoid scorpion Hemiscorpius lepturus where it represents 0.6% of the total protein content. It is a 33-mer basic peptide reticulated by three disulfide bridges, and that shares between 85 and 91% sequence identity with four other toxins, all known or supposed to be active on ryanodine-sensitive calcium channels. Hemicalcin differs from these other toxins by seven amino acids at positions 9 (leucine/arginine), 12 (alanine/glutamic acid), 13 (aspartic acid/asparagine), 14 (lysine/asparagine), 18 (serine/glycine), 26 (threonine/alanine) and 28 (proline/isoleucine/alanine). In spite of these differences, hemicalcin remains active on ryanodine-sensitive Ca2+ channels, since it increases [3H]ryanodine binding on RyR1 (ryanodine receptor type 1) and triggers Ca2+ release from sarcoplasmic vesicles. Bilayer lipid membrane experiments, in which the RyR1 channel is reconstituted and its gating properties are analysed, indicate that hemicalcin promotes an increase in the opening probability at intermediate concentration and induces a long-lasting subconductance level of 38% of the original amplitude at higher concentrations. Mice intracerebroventricular inoculation of 300 ng of hemicalcin induces neurotoxic symptoms in vivo, followed by death. Overall, these data identify a new biologically active toxin that belongs to a family of peptides active on the ryanodine-sensitive channel.
Figures


Similar articles
-
Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors.Br J Pharmacol. 2009 Jun;157(3):392-403. doi: 10.1111/j.1476-5381.2009.00147.x. Epub 2009 Apr 16. Br J Pharmacol. 2009. PMID: 19389159 Free PMC article.
-
The first report on transcriptome analysis of the venom gland of Iranian scorpion, Hemiscorpius lepturus.Toxicon. 2017 Jan;125:123-130. doi: 10.1016/j.toxicon.2016.11.261. Epub 2016 Nov 30. Toxicon. 2017. PMID: 27914888
-
Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca(2+) release channel/ryanodine receptors.FEBS Lett. 2000 Mar 10;469(2-3):179-85. doi: 10.1016/s0014-5793(00)01239-4. FEBS Lett. 2000. PMID: 10713267
-
A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran.Toxicon. 2010 Feb-Mar;55(2-3):173-9. doi: 10.1016/j.toxicon.2009.09.012. Epub 2009 Sep 30. Toxicon. 2010. PMID: 19799924 Review.
-
Scorpionism by Hemiscorpius spp. in Iran: a review.J Venom Anim Toxins Incl Trop Dis. 2018 Mar 2;24:8. doi: 10.1186/s40409-018-0145-z. eCollection 2018. J Venom Anim Toxins Incl Trop Dis. 2018. PMID: 29507581 Free PMC article. Review.
Cited by
-
Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor.Biophys J. 2008 Oct;95(7):3497-509. doi: 10.1529/biophysj.107.120840. Epub 2008 Jul 11. Biophys J. 2008. PMID: 18621823 Free PMC article.
-
Unique scorpion toxin with a putative ancestral fold provides insight into evolution of the inhibitor cystine knot motif.Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10478-83. doi: 10.1073/pnas.1103501108. Epub 2011 Jun 13. Proc Natl Acad Sci U S A. 2011. PMID: 21670253 Free PMC article.
-
The Effect of Hemiscorpius lepturus (Scorpionida: Hemiscorpiidae) Venom on Leukocytes and the Leukocyte Subgroups in Peripheral Blood of Rat.J Arthropod Borne Dis. 2016 Jan 5;10(2):159-67. eCollection 2016 Jun. J Arthropod Borne Dis. 2016. PMID: 27308274 Free PMC article.
-
Venoms of Iranian Scorpions (Arachnida, Scorpiones) and Their Potential for Drug Discovery.Molecules. 2019 Jul 23;24(14):2670. doi: 10.3390/molecules24142670. Molecules. 2019. PMID: 31340554 Free PMC article. Review.
-
Discovery of Leptulipin, a New Anticancer Protein from theIranian Scorpion, Hemiscorpius lepturus.Molecules. 2022 Mar 22;27(7):2056. doi: 10.3390/molecules27072056. Molecules. 2022. PMID: 35408455 Free PMC article.
References
-
- Radmanesh M. Clinical study of Hemiscorpion lepturus in Iran. J. Trop. Med. Hyg. 1990;93:327–332. - PubMed
-
- Franzini-Amstrong C., Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. - PubMed
-
- Leong P., MacLennan D. H. The cytoplasmic loops between domains II and III and domains III and IV in the skeletal muscle dihydropyridine receptor bind to a contiguous site in the skeletal muscle ryanodine receptor. J. Biol. Chem. 1998;273:29958–29964. - PubMed
-
- Zamudion Z. F., Conde R., Arévalo C., Beceril B., Martin M. B., Valvidia H. H., Possani L. D. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I, a heterodimeric protein from the scorpion Pandinus imperator. J. Biol. Chem. 1997;272:11886–11894. - PubMed
-
- El Hayek R., Lokuta A. J., Avrevalo C., Valvidia H. H. Peptide probe of ryanodine receptor function: imperatoxin A, a peptide from the venom of the scorpion Pandinus imperator, selectively activates skeletal-type ryanodine receptor isoforms. J. Biol. Chem. 1995;270:28696–28704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

