Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence
- PMID: 17291294
- PMCID: PMC3303600
- DOI: 10.1111/j.1474-9726.2007.00274.x
Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence
Abstract
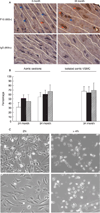
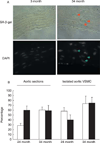
We previously reported that the frequency of polyploid aortic vascular smooth muscle cells (VSMC) serves as a biomarker of aging. Cellular senescence of somatic cells is another marker of aging that is characterized by the inability to undergo cell division. Here, we examined whether polyploidy is associated with the development of cellular senescence in vivo. Analysis of aortic tissue preparations from young and old Brown Norway rats showed that expression of senescence markers such as p16(INK4a) and senescence-associated beta-galactosidase activity are detected primarily in the old tissues. VSMC from p16(INK4a) knockout and control mice display similar levels of polyploid cells. Intriguingly, senescence markers are expressed in most, but not all, polyploid VSMC. Moreover, the polyploid cells exhibit limited proliferative capacity in comparison to their diploid counterparts. This study is the first to demonstrate in vivo that polyploid VSMC adopt a senescent phenotype.
Figures
Similar articles
-
Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy.Cell Cycle. 2009 Mar 15;8(6):902-8. doi: 10.4161/cc.8.6.7900. Epub 2009 Mar 21. Cell Cycle. 2009. PMID: 19221493 Free PMC article.
-
Effect of extracts from Radix Ginseng, Radix Notoginseng and Rhizoma Chuanxiong on delaying aging of vascular smooth muscle cells in aged rats.Chin J Integr Med. 2012 Aug;18(8):582-90. doi: 10.1007/s11655-012-1180-1. Epub 2012 Aug 2. Chin J Integr Med. 2012. PMID: 22855034
-
Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence.Biochem Biophys Res Commun. 2016 Sep 16;478(2):1006-13. doi: 10.1016/j.bbrc.2016.08.004. Epub 2016 Aug 3. Biochem Biophys Res Commun. 2016. PMID: 27498005
-
Vascular smooth muscle cell polyploidy: an adaptive or maladaptive response?J Cell Physiol. 2008 Jun;215(3):588-92. doi: 10.1002/jcp.21363. J Cell Physiol. 2008. PMID: 18181174 Review.
-
Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis.Cardiovasc Res. 2018 Mar 15;114(4):622-634. doi: 10.1093/cvr/cvy007. Cardiovasc Res. 2018. PMID: 29360955 Review.
Cited by
-
Features of senescence and cell death induced by doxorubicin in A549 cells: organization and level of selected cytoskeletal proteins.J Cancer Res Clin Oncol. 2010 May;136(5):717-36. doi: 10.1007/s00432-009-0711-4. Epub 2009 Nov 7. J Cancer Res Clin Oncol. 2010. PMID: 19898866 Free PMC article.
-
Oxidative stress-induced cellular senescence desensitizes cell growth and migration of vascular smooth muscle cells through down-regulation of platelet-derived growth factor receptor-beta.Aging (Albany NY). 2019 Oct 3;11(19):8085-8102. doi: 10.18632/aging.102270. Epub 2019 Oct 3. Aging (Albany NY). 2019. PMID: 31584878 Free PMC article.
-
Surface Lin28A expression consistent with cellular stress parallels indicators of senescence.Cardiovasc Res. 2023 May 2;119(3):743-758. doi: 10.1093/cvr/cvac122. Cardiovasc Res. 2023. PMID: 35880724 Free PMC article.
-
Collagenase-resistant collagen promotes mouse aging and vascular cell senescence.Aging Cell. 2014 Feb;13(1):121-30. doi: 10.1111/acel.12155. Epub 2013 Sep 19. Aging Cell. 2014. PMID: 23957394 Free PMC article.
-
Effects of donor age and proliferative aging on the phenotype stability of rat aortic smooth muscle cells.Physiol Rep. 2015 Nov;3(11):e12626. doi: 10.14814/phy2.12626. Epub 2015 Nov 24. Physiol Rep. 2015. PMID: 26603458 Free PMC article.
References
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
-
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. - PubMed
-
- Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb. Symp. Quant. Biol. 1994;59:67–73. - PubMed
-
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

