Utp14b: a unique retrogene within a gene that has acquired multiple promoters and a specific function in spermatogenesis
- PMID: 17291484
- PMCID: PMC1910592
- DOI: 10.1016/j.ydbio.2007.01.005
Utp14b: a unique retrogene within a gene that has acquired multiple promoters and a specific function in spermatogenesis
Abstract
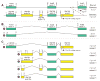
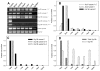
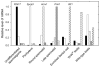
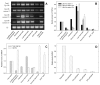
The mouse retrogene Utp14b is essential for male fertility, and a mutation in its sequence results in the sterile juvenile spermatogonial depletion (jsd) phenotype. It is a retrotransposed copy of the Utp14a gene, which is located on the X chromosome, and is inserted within an intron of the autosomal acyl-CoA synthetase long-chain family member 3 (Acsl3) gene. To elucidate the roles of the Utp14 genes in normal spermatogenic cell development as a basis for understanding the defects that result in the jsd phenotype, we analyzed the various mRNAs produced from the Utp14b retrogene and their expression in different cell types. Two classes of transcripts were identified: variant 1, a transcript driven by the host gene promoter, that is predominantly found in germ cells but is ubiquitously expressed at low levels; and variants 2-5, a group of alternatively spliced transcripts containing some unique untranslated exons that are transcribed from a novel promoter that is germ-cell-specific. Utp14b (predominantly variant 1) is expressed at moderately high levels in pachytene spermatocytes, the developmental stage at which the expression of the X-linked Utp14a is suppressed. The levels of both classes of Utp14b transcripts were highest in round spermatids despite the transcription of Utp14a in these cells. We propose that when Utp14b initially inserted into Acsl3, it utilized the Acsl3 promoter to drive expression in pachytene spermatocytes to compensate for inactivation of Utp14a expression. The novel cell-type-specific promoter for Utp14b likely evolved later, as the protein may have acquired a germ cell-specific function in spermatid development.
Figures



Similar articles
-
UTP14c is a recently acquired retrogene associated with spermatogenesis and fertility in man.Biol Reprod. 2006 Apr;74(4):644-51. doi: 10.1095/biolreprod.105.046698. Epub 2005 Dec 14. Biol Reprod. 2006. PMID: 16354793
-
The mouse juvenile spermatogonial depletion (jsd) phenotype is due to a mutation in the X-derived retrogene, mUtp14b.Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11695-700. doi: 10.1073/pnas.0401130101. Epub 2004 Aug 2. Proc Natl Acad Sci U S A. 2004. PMID: 15289605 Free PMC article.
-
An X-to-autosome retrogene is required for spermatogenesis in mice.Nat Genet. 2004 Aug;36(8):872-6. doi: 10.1038/ng1390. Epub 2004 Jul 18. Nat Genet. 2004. PMID: 15258580
-
Genetic factors contributing to defective spermatogonial differentiation in juvenile spermatogonial depletion (Utp14bjsd) mice.Biol Reprod. 2007 Aug;77(2):237-46. doi: 10.1095/biolreprod.107.060087. Epub 2007 May 2. Biol Reprod. 2007. PMID: 17475932
-
A model for understanding gene regulation during spermatogenesis: the mouse testis Pdha-2 promoter.Reprod Fertil Dev. 1995;7(4):705-12. doi: 10.1071/rd9950705. Reprod Fertil Dev. 1995. PMID: 8711207 Review.
Cited by
-
Increasing testicular temperature by exposure to elevated ambient temperatures restores spermatogenesis in adult Utp14b (jsd) mutant (jsd) mice.Andrology. 2015 Mar;3(2):376-84. doi: 10.1111/andr.287. Epub 2014 Oct 9. Andrology. 2015. PMID: 25303716 Free PMC article.
-
Androgen receptor in Sertoli cells is not required for testosterone-induced suppression of spermatogenesis, but contributes to Sertoli cell organization in Utp14b jsd mice.J Androl. 2009 May-Jun;30(3):338-48. doi: 10.2164/jandrol.108.006890. Epub 2009 Jan 8. J Androl. 2009. PMID: 19136388 Free PMC article.
-
Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice.Plant Cell. 2007 Aug;19(8):2329-48. doi: 10.1105/tpc.107.051300. Epub 2007 Aug 24. Plant Cell. 2007. PMID: 17720868 Free PMC article.
-
Analysis of DNA polymerase ν function in meiotic recombination, immunoglobulin class-switching, and DNA damage tolerance.PLoS Genet. 2017 Jun 1;13(6):e1006818. doi: 10.1371/journal.pgen.1006818. eCollection 2017 Jun. PLoS Genet. 2017. PMID: 28570559 Free PMC article.
-
Role of β-catenin in post-meiotic male germ cell differentiation.PLoS One. 2011;6(11):e28039. doi: 10.1371/journal.pone.0028039. Epub 2011 Nov 18. PLoS One. 2011. PMID: 22125654 Free PMC article.
References
-
- Ayoub N, Richler C, Wahrman J. Xist RNA is associated with the transcriptionally inactive XY body in mammalian male meiosis. Chromosoma. 1997;106:1–10. - PubMed
-
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–167. - PubMed
-
- Bedell MA, Mahakali Zama A. Genetic analysis of Kit ligand functions during mouse spermatogenesis. J Androl. 2004;25:188–199. - PubMed
-
- Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod. 2000;63:1185–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

