Chronic hypoxia in vivo reduces placental oxidative stress
- PMID: 17292468
- PMCID: PMC2001273
- DOI: 10.1016/j.placenta.2006.11.010
Chronic hypoxia in vivo reduces placental oxidative stress
Abstract
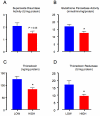
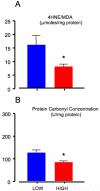
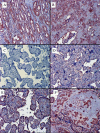
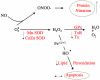
Decreased placental oxygenation and increased oxidative stress are implicated in the development of preeclampsia. Oxidative stress arises from imbalance between pro-versus anti-oxidants and can lead to biological oxidation and apoptosis. Because pregnant women living at high altitude (3100 m, HA) have lowered arterial PO2 and an increased incidence of preeclampsia, we hypothesized that HA placentas would have decreased anti-oxidant enzyme activity, increased oxidative stress (lipid peroxidation, protein oxidation and nitration) and greater trophoblast apoptosis than low-altitude (LA) placentas. We measured enzymatic activities, lipid and protein oxidation and co-factor concentrations by spectrophotometric techniques and ELISA in 12 LA and 18 HA placentas. Immunohistochemistry (IHC) was used to evaluate nitrated proteins and specific markers of apoptosis (activated caspase 3 and M30). Superoxide dismutase activity was marginally lower (p=0.05), while glutathione peroxidase activity (p<0.05), thioredoxin concentrations (p<0.005) and thioredoxin reductase activity p<0.01 were all reduced in HA placentas. Decreased anti-oxidant activity was not associated with increased oxidative stress: lipid peroxide content and protein carbonyl formation were lower at HA (p<0.01). We found greater nitrotyrosine residues in the syncytiotrophoblast at 3100 m (p<0.05), but apoptosis did not differ between altitudes. Our data suggest that hypoxia does not increase placental oxidative stress in vivo. Nitrative stress may be a consequence of hypoxia but does not appear to contribute to increased apoptosis. Lowered placental concentrations of anti-oxidants may contribute to the susceptibility of women living at HA to the development of preeclampsia, but are unlikely to be etiological.
Figures

Similar articles
-
SHH expression in placental tissues and trophoblast cell oxidative stress injury during preeclampsia.Eur Rev Med Pharmacol Sci. 2019 Jul;23(14):6026-6034. doi: 10.26355/eurrev_201907_18415. Eur Rev Med Pharmacol Sci. 2019. PMID: 31364136
-
Apelin/APJ system protects placental trophoblasts from hypoxia-induced oxidative stress through activating PI3K/Akt signaling pathway in preeclampsia.Free Radic Biol Med. 2023 Nov 1;208:759-770. doi: 10.1016/j.freeradbiomed.2023.09.030. Epub 2023 Sep 27. Free Radic Biol Med. 2023. PMID: 37774802
-
Oxidative stress contributes to hypermethylation of Histone H3 lysine 9 in placental trophoblasts from preeclamptic pregnancies.Front Endocrinol (Lausanne). 2024 Apr 25;15:1371220. doi: 10.3389/fendo.2024.1371220. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38737551 Free PMC article.
-
Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta.Placenta. 2010 Mar;31 Suppl(Suppl):S66-9. doi: 10.1016/j.placenta.2009.12.021. Epub 2010 Jan 27. Placenta. 2010. PMID: 20110125 Free PMC article. Review.
-
Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction.Hum Reprod Update. 2021 Apr 21;27(3):531-569. doi: 10.1093/humupd/dmaa053. Hum Reprod Update. 2021. PMID: 33377492 Review.
Cited by
-
Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy.Hum Reprod Update. 2010 Jul-Aug;16(4):415-31. doi: 10.1093/humupd/dmp046. Epub 2009 Nov 19. Hum Reprod Update. 2010. PMID: 19926662 Free PMC article. Review.
-
Oxygen, the Janus gas; its effects on human placental development and function.J Anat. 2009 Jul;215(1):27-35. doi: 10.1111/j.1469-7580.2008.00978.x. Epub 2008 Oct 13. J Anat. 2009. PMID: 19175804 Free PMC article. Review.
-
Mitochondrial Dysfunction in the Pathogenesis of Preeclampsia.Curr Hypertens Rep. 2022 Jun;24(6):157-172. doi: 10.1007/s11906-022-01184-7. Epub 2022 Mar 7. Curr Hypertens Rep. 2022. PMID: 35254588 Free PMC article. Review.
-
Effect of Oxidative Stress on the Estrogen-NOS-NO-KCa Channel Pathway in Uteroplacental Dysfunction: Its Implication in Pregnancy Complications.Oxid Med Cell Longev. 2019 Feb 10;2019:9194269. doi: 10.1155/2019/9194269. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30881600 Free PMC article. Review.
-
Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy.J Physiol. 2016 Mar 1;594(5):1371-88. doi: 10.1113/JP271073. Epub 2015 Sep 15. J Physiol. 2016. PMID: 26278110 Free PMC article.
References
-
- Mahfouz AAR, El-Aid MM, Alakija W, Al-Erian RAG. Altitude and socio-biological determinants of pregnancy-associated hypertension. Int J. Obstet Gynecol. 1994;44:135–138. - PubMed
-
- Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–8. - PubMed
-
- Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54:20–5. - PubMed
-
- Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–91. - PubMed
-
- Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. 1995;79:7–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

