Characterization of methyl-beta-cyclodextrin toxicity in NGF-differentiated PC12 cell death
- PMID: 17292476
- PMCID: PMC1994916
- DOI: 10.1016/j.neuro.2007.01.001
Characterization of methyl-beta-cyclodextrin toxicity in NGF-differentiated PC12 cell death
Abstract
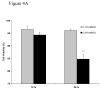
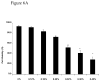
Cyclodextrins (CDs) are used to deliver hydrophobic molecules in aqueous environments. Methyl-beta-cyclodextrin (MbetaCD), a member of this family of molecules, has been proposed to be a good carrier to deliver fatty acids to cells in culture. This report focuses on studying the in vitro effects of MbetaCD on nerve growth factor-differentiated PC12 (NGFDPC12) cells, a tissue culture model to study neuronal survival and differentiation. The main findings are: (1) NGFDPC12 cells have normal viability when exposed to 0.12% MbetaCD but showed a significant loss in cell viability at higher concentrations; (2) NGFDPC12 cells exposed to 0.25% MbetaCD exhibit nuclear condensation, blebbing and apoptotic bodies, and whole cell lysates exhibited an increase in caspase-3-like activity and high levels of Bax and Bcl-X(L) protein expression compared to control. Cultures treated with 0.25% MbetaCD also showed cleavage of normal 21-kDa Bax protein into a 18-kDa fragment. (3) Experiments using 0.12% MbetaCD to deliver oleic acid did not affect cell viability, in contrast NGFDPC12 cultures in which 0.25% MbetaCD concentration is used exhibited similar loss of cell viability as observed with 0.25% MbetaCD alone. Treating these cultures with caspase-3 inhibitor z-VAD-fmk did not protect the cells from MbetaCD toxic effects. (4) Immortalized Schwann cells (iSC) exposed to MbetaCD 0.12% did not show loss of cell viability while 0.25% MbetaCD triggered a significant toxicity but with a different dose and time course dynamic than NGFDPC12 cells. Thus, NGFDPC12 or iSC cell cultures exposed to 0.12% MbetaCD exhibits normal viability while higher concentrations increase in cell death and apoptosis.
Figures










Similar articles
-
Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells.J Neurochem. 2003 Feb;84(4):655-68. doi: 10.1046/j.1471-4159.2003.01571.x. J Neurochem. 2003. PMID: 12562510 Free PMC article.
-
Docosahexaenoic acid protection against palmitic acid-induced lipotoxicity in NGF-differentiated PC12 cells involves enhancement of autophagy and inhibition of apoptosis and necroptosis.J Neurochem. 2020 Dec;155(5):559-576. doi: 10.1111/jnc.15038. Epub 2020 Jun 8. J Neurochem. 2020. PMID: 32379343 Free PMC article.
-
Expression of E-FABP in PC12 cells increases neurite extension during differentiation: involvement of n-3 and n-6 fatty acids.J Neurochem. 2008 Sep;106(5):2015-29. doi: 10.1111/j.1471-4159.2008.05507.x. Epub 2008 May 30. J Neurochem. 2008. PMID: 18513372 Free PMC article.
-
Epidermal fatty acid-binding protein protects nerve growth factor-differentiated PC12 cells from lipotoxic injury.J Neurochem. 2015 Jan;132(1):85-98. doi: 10.1111/jnc.12934. Epub 2014 Sep 19. J Neurochem. 2015. PMID: 25147052 Free PMC article.
-
Nerve growth factor increases the sensitivity to zinc toxicity and induces cell cycle arrest in PC12 cells.Brain Res Bull. 2010 Mar 16;81(4-5):458-66. doi: 10.1016/j.brainresbull.2009.11.008. Epub 2009 Dec 1. Brain Res Bull. 2010. PMID: 19931600
Cited by
-
Saturated Fatty Acids Induce Ceramide-associated Macrophage Cell Death.J Vis Exp. 2017 Oct 31;(128):56535. doi: 10.3791/56535. J Vis Exp. 2017. PMID: 29155746 Free PMC article.
-
Dietary Docosahexaenoic Acid-Rich Supplementation Decreases Neurotoxic Lipid Mediators in Participants with Type 2 Diabetes and Neuropathic Pain.Nutrients. 2024 Nov 24;16(23):4025. doi: 10.3390/nu16234025. Nutrients. 2024. PMID: 39683418 Free PMC article.
-
Defining Lineage-Specific Membrane Fluidity Signatures that Regulate Adhesion Kinetics.Stem Cell Reports. 2018 Oct 9;11(4):852-860. doi: 10.1016/j.stemcr.2018.08.010. Epub 2018 Sep 6. Stem Cell Reports. 2018. PMID: 30197117 Free PMC article.
-
Significantly improving the solubility and anti-inflammatory activity of fenofibric acid with native and methyl-substituted beta-cyclodextrins via complexation.Sci Rep. 2025 Jan 5;15(1):853. doi: 10.1038/s41598-024-84745-x. Sci Rep. 2025. PMID: 39757237 Free PMC article.
-
The Role of Cyclodextrins in the Design and Development of Triterpene-Based Therapeutic Agents.Int J Mol Sci. 2022 Jan 10;23(2):736. doi: 10.3390/ijms23020736. Int J Mol Sci. 2022. PMID: 35054925 Free PMC article. Review.
References
-
- Adams JM, Cory S. The bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1325. - PubMed
-
- Bar-On P, Rockenstein E, Adame A, Ho G, Hashimoto M, Masliah E. Effect of the cholesterol-lowering compound methyl-β-cyclodextrin in models of -synucleinopathy. 2006;98:1032–1045. - PubMed
-
- Bender ML, Komiyama M. Cyclodextrin Chemistry. Heidelberg: Springer-Verlag; 1978. pp. 10–27.
-
- Bernard DW, Rodriguez A, Rothblat GH, Glick JM. Influence of high density lipoprotein on esterified cholesterol stores in macrophages and hepatoma cells. Arteriosclerosis. 1990;10:135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

