CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy
- PMID: 17292584
- PMCID: PMC2062574
- DOI: 10.1016/j.bbi.2006.12.003
CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy
Abstract
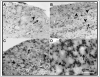
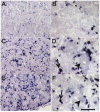
Nucleoside reverse transcriptase inhibitors (NRTIs) are known to produce painful neuropathies and to enhance states of pain hypersensitivity produced by HIV-1 infection. It has also been observed that in some neuropathic pain models, chemokines and their receptors are upregulated, perhaps contributing to the pain state. In order to understand if chemokines are involved in NRTI-mediated sensory neuropathies, we treated rats with the anti-retroviral drug, 2',3'-dideoxycytidine (ddC), which is known to produce an extended period of hyperalgesia and allodynia. Using in situ hybridization, we observed that under normal conditions, CXCR4 chemokine receptors were widely expressed by satellite glia in the dorsal root ganglia (DRG) and Schwann cells in the sciatic nerve. A limited number of DRG neurons also expressed CXCR4 receptors. The chemokine SDF-1/CXCL12 was similarly expressed in glial cells in the DRG and peripheral nerve. Following a single administration of ddC, expression levels of CXCR4 mRNA in glia and neurons and SDF-1 mRNA in glia increased considerably. The functional nature of increased CXCR4 mRNA expression was confirmed by measuring SDF-1 induced [Ca2+]i increases in acutely isolated DRG neurons and glia. In contrast, the expression of the chemokine receptors CCR2 and CCR5 did not change following ddC treatment. Pain hypersensitivity produced by ddC could be inhibited by treatment with the CXCR4 antagonist, AMD3100. Hence, we postulate that NRTIs produce pain hypersensitivity through the upregulation of CXCR4 signaling in the DRG. Increased numbers of CXCR4 receptors would also explain the synergism observed between NRTI treatment and the proalgesic effects of HIV-1 infection.
Figures




References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Berger JR, Levy RM. The neurologic complications of human immunodeficiency virus infection. Med Clin North Am. 1993;77:1–23. - PubMed
-
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

