Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C
- PMID: 17293397
- PMCID: PMC1852344
- DOI: 10.1529/biophysj.106.095406
Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C
Abstract
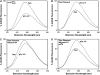
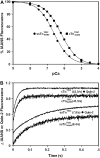
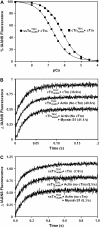
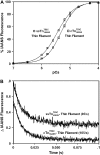
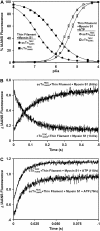
Understanding the effects of thin and thick filament proteins on the kinetics of Ca(2+) exchange with cardiac troponin C is essential to elucidating the Ca(2+)-dependent mechanisms controlling cardiac muscle contraction and relaxation. Unlike labeling of the endogenous Cys-84, labeling of cardiac troponin C at a novel engineered Cys-53 with 2-(4'-iodoacetamidoanilo)napthalene-6-sulfonic acid allowed us to accurately measure the rate of calcium dissociation from the regulatory domain of troponin C upon incorporation into the troponin complex. Neither tropomyosin nor actin alone affected the Ca(2+) binding properties of the troponin complex. However, addition of actin-tropomyosin to the troponin complex decreased the Ca(2+) sensitivity ( approximately 7.4-fold) and accelerated the rate of Ca(2+) dissociation from the regulatory domain of troponin C ( approximately 2.5-fold). Subsequent addition of myosin S1 to the reconstituted thin filaments (actin-tropomyosin-troponin) increased the Ca(2+) sensitivity ( approximately 6.2-fold) and decreased the rate of Ca(2+) dissociation from the regulatory domain of troponin C ( approximately 8.1-fold), which was completely reversed by ATP. Consistent with physiological data, replacement of cardiac troponin I with slow skeletal troponin I led to higher Ca(2+) sensitivities and slower Ca(2+) dissociation rates from troponin C in all the systems studied. Thus, both thin and thick filament proteins influence the ability of cardiac troponin C to sense and respond to Ca(2+). These results imply that both cross-bridge kinetics and Ca(2+) dissociation from troponin C work together to modulate the rate of cardiac muscle relaxation.
Figures

References
-
- Kobayashi, T., and R. J. Solaro. 2005. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu. Rev. Physiol. 67:39–67. - PubMed
-
- Gordon, A. M., E. Homsher, and M. Regnier. 2000. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924. - PubMed
-
- Tikunova, S. B., J. A. Rall, and J. P. Davis. 2002. Effect of hydrophobic residue substitutions with glutamine on Ca(2+) binding and exchange with the N-domain of troponin C. Biochemistry. 41:6697–6705. - PubMed
-
- Dong, W. J., J. M. Robinson, J. Xing, and H. C. Cheung. 2003. Kinetics of conformational transitions in cardiac troponin induced by Ca2+ dissociation determined by Forster resonance energy transfer. J. Biol. Chem. 278:42394–42402. - PubMed
-
- Johnson, J. D., J. H. Collins, S. P. Robertson, and J. D. Potter. 1980. A fluorescent probe study of Ca2+ binding to the Ca2+-specific sites of cardiac troponin and troponin C. J. Biol. Chem. 255:9635–9640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

