Calorimetric, x-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes
- PMID: 17293402
- PMCID: PMC1852355
- DOI: 10.1529/biophysj.106.094003
Calorimetric, x-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes
Abstract
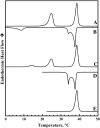
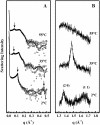
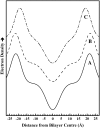
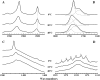
The thermotropic phase behavior and organization of aqueous dispersions of the quadruple-chained, anionic phospholipid tetramyristoyl diphosphatidylglycerol or tetramyristoyl cardiolipin (TMCL) was studied by differential scanning calorimetry, x-ray diffraction, (31)P NMR, and Fourier-transform infrared (FTIR) spectroscopy. At physiological pH and ionic strength, our calorimetric studies indicate that fully equilibrated aqueous dispersions of TMCL exhibit two thermotropic phase transitions upon heating. The lower temperature transition is much less cooperative but of relatively high enthalpy and exhibits marked cooling hysteresis, whereas the higher temperature transition is much more cooperative and also exhibits a relatively high enthalpy but with no appreciable cooling hysteresis. Also, the properties of these two-phase transitions are sensitive to the ionic strength of the dispersing buffer. Our spectroscopic and x-ray diffraction data indicate that the lower temperature transition corresponds to a lamellar subgel (L(c)') to gel (L(beta)) phase transition and the higher temperature endotherm to a L(beta) to lamellar liquid-crystalline (L(alpha)) phase transition. At the L(c)'/L(beta) phase transition, there is a fivefold increase of the thickness of the interlamellar aqueous space from approximately 11 A to approximately 50 A, and this value decreases slightly at the L(beta)/L(alpha) phase transition. The bilayer thickness (i.e., the mean phosphate-phosphate distance across the bilayer) increases from 42.8 A to 43.5 A at the L(c)'/L(beta) phase transition, consistent with the loss of the hydrocarbon chain tilt of approximately 12 degrees , and decreases to 37.8 A at the L(beta)/L(alpha) phase transition. The calculated cross-sectional areas of the TMCL molecules are approximately 79 A(2) and approximately 83 A(2) in the L(c)' and L(beta) phases, respectively, and we estimate a value of approximately 100 A(2) in the L(alpha) phase. The combination of x-ray and FTIR spectroscopic data indicate that in the L(c)' phase, TMCL molecules possess tilted all-trans hydrocarbon chains packed into an orthorhombic subcell in which the zig-zag planes of the chains are parallel, while in the L(beta) phase the untilted, all-trans hydrocarbon chains possess rotational mobility and are packed into a hexagonal subcell, as are the conformationally disordered hydrocarbon chains in the L(alpha) phase. Our FTIR spectroscopic results demonstrate that the four carbonyl groups of the TMCL molecule become progressively more hydrated as one proceeds from the L(c)' to the L(beta) and then to the L(alpha) phase, while the two phosphate moieties of the polar headgroup are comparably well hydrated in all three phases. Our (31)P-NMR results indicate that although the polar headgroup retains some mobility in the L(c)' phase, its motion is much more restricted in the L(beta) and especially in the L(alpha) phase than that of other phospholipids. We can explain most of our experimental results on the basis of the relatively small size of the polar headgroup of TMCL relative to other phospholipids and the covalent attachment of the two phosphate moieties to a single glycerol moiety, which results in a partially immobilized polar headgroup that is more exposed to the solvent than in other glycerophospholipids. Finally, we discuss the biological relevance of the unique properties of TMCL to the structure and function of cardiolipin-containing biological membranes.
Figures


References
-
- Daum, G. 1985. Lipids of mitochondria. Biochim. Biophys. Acta. 822:1–42. - PubMed
-
- Ratledge, C., and S. G. Wilkinson. 1988. Fatty acids, related and derived lipids. In Microbial Lipids. C. Ratledge and S. G. Wilkinson, editors. Academic Press, London.
-
- O'Leary, W. M., and S. G. Wilkinson. 1988. Gram-positive bacteria. In Microbial Lipids. C. Ratledge and S. G. Wilkinson, editors. Academic Press, London.
-
- Wilkinson, S. G. 1988. Gram-negative bacteria. In Microbial Lipids. C. Ratledge and S. G. Wilkinson, editors. Academic Press, London.
-
- Hoch, F. L. 1992. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1113:71–133. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

