Compartmentation of cAMP signaling in cardiac myocytes: a computational study
- PMID: 17293406
- PMCID: PMC1852367
- DOI: 10.1529/biophysj.106.095356
Compartmentation of cAMP signaling in cardiac myocytes: a computational study
Erratum in
- Biophys J. 2008 Jan 15;94(2):714
Abstract
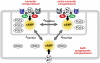
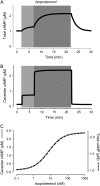
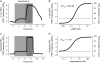
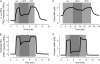
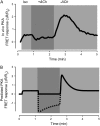
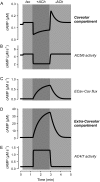
Receptor-mediated changes in cAMP production play an essential role in sympathetic and parasympathetic regulation of the electrical, mechanical, and metabolic activity of cardiac myocytes. However, responses to receptor activation cannot be easily ascribed to a uniform increase or decrease in cAMP activity throughout the entire cell. In this study, we used a computational approach to test the hypothesis that in cardiac ventricular myocytes the effects of beta(1)-adrenergic receptor (beta(1)AR) and M(2) muscarinic receptor (M(2)R) activation involve compartmentation of cAMP. A model consisting of two submembrane (caveolar and extracaveolar) microdomains and one bulk cytosolic domain was created using published information on the location of beta(1)ARs and M(2)Rs, as well as the location of stimulatory (G(s)) and inhibitory (G(i)) G-proteins, adenylyl cyclase isoforms inhibited (AC5/6) and stimulated (AC4/7) by G(i), and multiple phosphodiesterase isoforms (PDE2, PDE3, and PDE4). Results obtained with the model indicate that: 1), bulk basal cAMP can be high ( approximately 1 microM) and only modestly stimulated by beta(1)AR activation ( approximately 2 microM), but caveolar cAMP varies in a range more appropriate for regulation of protein kinase A ( approximately 100 nM to approximately 2 microM); 2), M(2)R activation strongly reduces the beta(1)AR-induced increases in caveolar cAMP, with less effect on bulk cAMP; and 3), during weak beta(1)AR stimulation, M(2)R activation not only reduces caveolar cAMP, but also produces a rebound increase in caveolar cAMP following termination of M(2)R activity. We conclude that compartmentation of cAMP can provide a quantitative explanation for several aspects of cardiac signaling.
Figures
References
-
- Kameyama, M., F. Hofmann, and W. Trautwein. 1985. On the mechanism of β-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 405:285–293. - PubMed
-
- Terasaki, W. L., and G. Brooker. 1977. Cardiac adenosine 3′:5′-monophosphate. Free and bound forms in the isolated rat atrium. J. Biol. Chem. 252:1041–1050. - PubMed
-
- Mongillo, M., T. McSorley, S. Evellin, A. Sood, V. Lissandron, A. Terrin, E. Huston, A. Hannawacker, M. J. Lohse, T. Pozzan, M. D. Houslay, and M. Zaccolo. 2004. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ. Res. 95:67–75. - PubMed
-
- Adams, S. R., A. T. Harootunian, Y. J. Buechler, S. S. Taylor, and R. Y. Tsien. 1991. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 349:694–697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

