Channel opening by anesthetics and GABA induces similar changes in the GABAA receptor M2 segment
- PMID: 17293408
- PMCID: PMC1852347
- DOI: 10.1529/biophysj.106.094490
Channel opening by anesthetics and GABA induces similar changes in the GABAA receptor M2 segment
Abstract
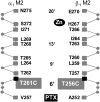
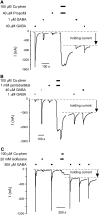
For many general anesthetics, their molecular basis of action involves interactions with GABA(A) receptors. Anesthetics produce concentration-dependent effects on GABA(A) receptors. Low concentrations potentiate submaximal GABA-induced currents. Higher concentrations directly activate the receptors. Functional effects of anesthetics have been characterized, but little is known about the conformational changes they induce. We probed anesthetic-induced conformational changes in the M2 membrane-spanning, channel-lining segment using disulfide trapping between engineered cysteines. Previously, we showed that oxidation by copper phenanthroline in the presence of GABA of the M2 6' cysteine mutants, alpha(1)T261Cbeta(1)T256C and alpha(1)beta(1)T256C resulted in formation of an intersubunit disulfide bond between the adjacent beta-subunits that significantly increased the channels' spontaneous open probability. Oxidation in GABA's absence had no effect. We examined the effect on alpha(1)T261Cbeta(1)T256C and on alpha(1)beta(1)T256C of oxidation by copper phenanthroline in the presence of potentiating and directly activating concentrations of the general anesthetics propofol, pentobarbital, and isoflurane. Oxidation in the presence of potentiating concentration of anesthetics had little effect. Oxidation in the presence of directly activating anesthetic concentrations significantly increased the channels' spontaneous open probability. We infer that activation by anesthetics and GABA induces a similar conformational change at the M2 segment 6' position that is related to channel opening.
Figures



Similar articles
-
Structural evidence that propofol stabilizes different GABA(A) receptor states at potentiating and activating concentrations.J Neurosci. 2002 Sep 1;22(17):7417-24. doi: 10.1523/JNEUROSCI.22-17-07417.2002. J Neurosci. 2002. PMID: 12196563 Free PMC article.
-
Modulation of GABA(A) receptor channel gating by pentobarbital.J Physiol. 2001 Dec 15;537(Pt 3):715-33. doi: 10.1111/j.1469-7793.2001.00715.x. J Physiol. 2001. PMID: 11744750 Free PMC article.
-
Coadministered nitrous oxide enhances the effect of isoflurane on GABAergic transmission by an increase in open-channel block.J Pharmacol Exp Ther. 2001 Jul;298(1):201-8. J Pharmacol Exp Ther. 2001. PMID: 11408543
-
Mapping General Anesthetic Sites in Heteromeric γ-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes.Anesth Analg. 2016 Nov;123(5):1263-1273. doi: 10.1213/ANE.0000000000001368. Anesth Analg. 2016. PMID: 27167687 Free PMC article. Review.
-
The molecular mechanism of action of general anesthetics: structural aspects of interactions with GABA(A) receptors.Toxicol Lett. 1998 Nov 23;100-101:193-201. doi: 10.1016/s0378-4274(98)00185-4. Toxicol Lett. 1998. PMID: 10049142 Review.
Cited by
-
Shared structural mechanisms of general anaesthetics and benzodiazepines.Nature. 2020 Sep;585(7824):303-308. doi: 10.1038/s41586-020-2654-5. Epub 2020 Sep 2. Nature. 2020. PMID: 32879488 Free PMC article.
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
The effect of pentobarbital sodium and propofol anesthesia on multifocal electroretinograms in rhesus macaques.Doc Ophthalmol. 2012 Feb;124(1):59-72. doi: 10.1007/s10633-011-9306-x. Epub 2011 Dec 27. Doc Ophthalmol. 2012. PMID: 22200766 Free PMC article.
-
Identification of an Inhibitory Alcohol Binding Site in GABAA ρ1 Receptors.ACS Chem Neurosci. 2016 Jan 20;7(1):100-8. doi: 10.1021/acschemneuro.5b00246. Epub 2015 Nov 25. ACS Chem Neurosci. 2016. PMID: 26571107 Free PMC article.
-
Crosslinking constraints and computational models as complementary tools in modeling the extracellular domain of the glycine receptor.PLoS One. 2014 Jul 15;9(7):e102571. doi: 10.1371/journal.pone.0102571. eCollection 2014. PLoS One. 2014. PMID: 25025226 Free PMC article.
References
-
- Rudolph, U., and B. Antkowiak. 2004. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 5:709–720. - PubMed
-
- Jurd, R., M. Arras, S. Lambert, B. Drexler, R. Siegwart, F. Crestani, M. Zaugg, K. E. Vogt, B. Ledermann, B. Antkowiak, and U. Rudolph. 2003. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor β3 subunit. FASEB J. 17:250–252. - PubMed
-
- Franks, N. P., and W. R. Lieb. 1998. Which molecular targets are most relevant to general anaesthesia? Toxicol. Lett. 100–101:1–8. - PubMed
-
- Evers, A. S., and J. H. Steinbach. 1999. Double-edged swords: volatile anesthetics both enhance and inhibit ligand-gated ion channels. Anesthesiology. 90:1–3. - PubMed
-
- Hemmings, H. C., Jr., M. H. Akabas, P. A. Goldstein, J. R. Trudell, B. A. Orser, and N. L. Harrison. 2005. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 26:503–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

