Physiological effects of Crl in Salmonella are modulated by sigmaS level and promoter specificity
- PMID: 17293430
- PMCID: PMC1855858
- DOI: 10.1128/JB.01919-06
Physiological effects of Crl in Salmonella are modulated by sigmaS level and promoter specificity
Abstract
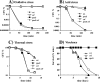
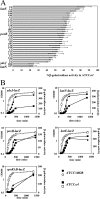
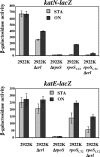
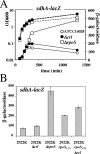
The small regulatory protein Crl activates sigma(S) (RpoS), the stationary-phase and general stress response sigma factor. Crl has been reported to bind sigma(S) in vitro and to facilitate the formation of RNA polymerase holoenzyme. In Salmonella enterica serovar Typhimurium, Crl is required for the development of the rdar morphotype and transcription initiation of the sigma(S)-dependent genes csgD and adrA, involved in curli and cellulose production. Here, we examined the expression of other sigma(S)-dependent phenotypes and genes in a Deltacrl mutant of Salmonella. Gene fusion analyses and in vitro transcription assays indicate that the magnitude of Crl activation differs between promoters and is highly dependent on sigma(S) levels. We replaced the wild-type rpoS allele in S. enterica serovar Typhimurium strain ATCC 14028 with the rpoS(LT2) allele that shows reduced expression of sigma(S); the result was an increased Crl activation ratio and larger physiological effects of Crl on oxidative, thermal, and acid stress resistance levels during stationary phase. We also found that crl, rpoS, and crl rpoS strains grew better on succinate than did the wild type and expressed the succinate dehydrogenase sdhCDBA operon more strongly. The crl and rpoS(LT2) mutations also increased the competitive fitness of Salmonella in stationary phase. These results show that Crl contributes to negative regulation by sigma(S), a finding consistent with a role for Crl in sigma factor competition via the facilitation of sigma(S) binding to core RNA polymerase.
Figures




Similar articles
-
Crl activates transcription initiation of RpoS-regulated genes involved in the multicellular behavior of Salmonella enterica serovar Typhimurium.J Bacteriol. 2006 Jun;188(11):3983-94. doi: 10.1128/JB.00033-06. J Bacteriol. 2006. PMID: 16707690 Free PMC article.
-
Crl binds to domain 2 of σ(S) and confers a competitive advantage on a natural rpoS mutant of Salmonella enterica serovar Typhi.J Bacteriol. 2010 Dec;192(24):6401-10. doi: 10.1128/JB.00801-10. Epub 2010 Oct 8. J Bacteriol. 2010. PMID: 20935100 Free PMC article.
-
Identification of conserved amino acid residues of the Salmonella sigmaS chaperone Crl involved in Crl-sigmaS interactions.J Bacteriol. 2010 Feb;192(4):1075-87. doi: 10.1128/JB.01197-09. Epub 2009 Dec 11. J Bacteriol. 2010. PMID: 20008066 Free PMC article.
-
Recent advances in the characterization of Crl, the unconventional activator of the stress sigma factor σS/RpoS.Biomol Concepts. 2016 Jun 1;7(3):197-204. doi: 10.1515/bmc-2016-0006. Biomol Concepts. 2016. PMID: 27180360 Review.
-
Regulation of biofilm formation in Salmonella enterica serovar Typhimurium.Future Microbiol. 2014;9(11):1261-82. doi: 10.2217/fmb.14.88. Future Microbiol. 2014. PMID: 25437188 Review.
Cited by
-
Gene order and chromosome dynamics coordinate spatiotemporal gene expression during the bacterial growth cycle.Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):E42-50. doi: 10.1073/pnas.1108229109. Epub 2011 Dec 19. Proc Natl Acad Sci U S A. 2012. PMID: 22184251 Free PMC article.
-
Effect of growth temperature on Crl-dependent regulation of sigmaS activity in Salmonella enterica serovar Typhimurium.J Bacteriol. 2008 Jul;190(13):4453-9. doi: 10.1128/JB.00154-08. Epub 2008 May 2. J Bacteriol. 2008. PMID: 18456810 Free PMC article.
-
A negative feedback loop is critical for recovery of RpoS after stress in Escherichia coli.PLoS Genet. 2024 Mar 11;20(3):e1011059. doi: 10.1371/journal.pgen.1011059. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38466775 Free PMC article.
-
Repressor activity of the RpoS/σS-dependent RNA polymerase requires DNA binding.Nucleic Acids Res. 2015 Feb 18;43(3):1456-68. doi: 10.1093/nar/gku1379. Epub 2015 Jan 10. Nucleic Acids Res. 2015. PMID: 25578965 Free PMC article.
-
Loss of CorA, the primary magnesium transporter of Salmonella, is alleviated by MgtA and PhoP-dependent compensatory mechanisms.PLoS One. 2023 Sep 15;18(9):e0291736. doi: 10.1371/journal.pone.0291736. eCollection 2023. PLoS One. 2023. PMID: 37713445 Free PMC article.
References
-
- Bang, I.-S., J. G. Frye, M. McClelland, J. Velayudhan, and F. C. Fang. 2005. Alternative sigma factor interactions in Salmonella: σE and σH promote antioxidant defences by enhancing σS levels. Mol. Microbiol. 56:811-823. - PubMed
-
- Bougdour, A., C. Lelong, and J. Geiselmann. 2004. Crl, a low temperature-induced protein in Escherichia coli that binds directly to the stationary phase sigma subunit of RNA polymerase. J. Biol. Chem. 279:19540-19550. - PubMed
-
- Chen, G., C. L. Patten, and H. E. Schellhorn. 2004. Positive selection for loss of RpoS function in Escherichia coli. Mutat. Res. 554:193-203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

