Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco
- PMID: 17293434
- PMCID: PMC1851823
- DOI: 10.1104/pp.106.094771
Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco
Abstract
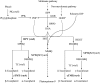
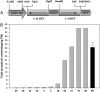
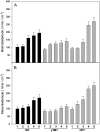
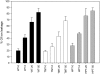
Tocopherols are lipophilic antioxidants that are synthesized exclusively in photosynthetic organisms. In most higher plants, alpha- and gamma-tocopherol are predominant with their ratio being under spatial and temporal control. While alpha-tocopherol accumulates predominantly in photosynthetic tissue, seeds are rich in gamma-tocopherol. To date, little is known about the specific roles of alpha- and gamma-tocopherol in different plant tissues. To study the impact of tocopherol composition and content on stress tolerance, transgenic tobacco (Nicotiana tabacum) plants constitutively silenced for homogentisate phytyltransferase (HPT) and gamma-tocopherol methyltransferase (gamma-TMT) activity were created. Silencing of HPT lead to an up to 98% reduction of total tocopherol accumulation compared to wild type. Knockdown of gamma-TMT resulted in an up to 95% reduction of alpha-tocopherol in leaves of the transgenics, which was almost quantitatively compensated for by an increase in gamma-tocopherol. The response of HPT and gamma-TMT transgenics to salt and sorbitol stress and methyl viologen treatments in comparison to wild type was studied. Each stress condition imposes oxidative stress along with additional challenges like perturbing ion homeostasis, desiccation, or disturbing photochemistry, respectively. Decreased total tocopherol content increased the sensitivity of HPT:RNAi transgenics toward all tested stress conditions, whereas gamma-TMT-silenced plants showed an improved performance when challenged with sorbitol or methyl viologen. However, salt tolerance of gamma-TMT transgenics was strongly decreased. Membrane damage in gamma-TMT transgenic plants was reduced after sorbitol and methyl viologen-mediated stress, as evident by less lipid peroxidation and/or electrolyte leakage. Therefore, our results suggest specific roles for alpha- and gamma-tocopherol in vivo.
Figures




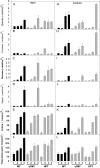
References
-
- Alscher RGEN, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53 1331–1341 - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 403–410 - PubMed
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/ H+ antiporter in Arabidopsis. Science 285 1256–1258 - PubMed
-
- Baroli I, Gutman BL, Ledford HK, Shin JW, Chin BL, Havaux M, Niyogi KK (2004) Photo-oxidative stress in a xanthophyll-deficient mutant of Chlamydomonas. J Biol Chem 279 6337–6344 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

