Campylobacter jejuni strains compete for colonization in broiler chicks
- PMID: 17293510
- PMCID: PMC1855682
- DOI: 10.1128/AEM.02193-06
Campylobacter jejuni strains compete for colonization in broiler chicks
Abstract
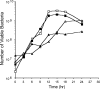
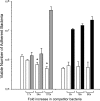
Campylobacter jejuni isolates possess multiple adhesive proteins termed adhesins, which promote the organism's attachment to epithelial cells. Based on the proposal that one or more adhesins are shared among C. jejuni isolates, we hypothesized that C. jejuni strains would compete for intestinal and cecal colonization in broiler chicks. To test this hypothesis, we selected two C. jejuni strains with unique SmaI pulsed-field gel electrophoresis macrorestriction profiles and generated one nalidixic acid-resistant strain (the F38011 Nal(r) strain) and one streptomycin-resistant strain (the 02-833L Str(r) strain). In vitro binding assays revealed that the C. jejuni F38011 Nal(r) and 02-833L Str(r) strains adhered to LMH chicken hepatocellular carcinoma epithelial cells and that neither strain influenced the binding potential of the other strain at low inoculation doses. However, an increase in the dose of the C. jejuni 02-833L Str(r) strain relative to that of the C. jejuni F38011 Nal(r) strain competitively inhibited the binding of the C. jejuni F38011 Nal(r) strain to LMH cells in a dose-dependent fashion. Similarly, the C. jejuni 02-833L Str(r) strain was found to significantly reduce the efficiency of intestinal and cecal colonization by the C. jejuni F38011 Nal(r) strain in broiler chickens. Based on the number of bacteria recovered from the ceca, the maximum number of bacteria that can colonize the digestive tracts of chickens may be limited by host constraints. Collectively, these data support the hypothesis that C. jejuni strains compete for colonization in chicks and suggest that it may be possible to design novel intervention strategies for reducing the level at which C. jejuni colonizes the cecum.
Figures






Similar articles
-
The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein.Avian Dis. 1999 Jul-Sep;43(3):586-9. Avian Dis. 1999. PMID: 10494431
-
Role of Campylobacter jejuni potential virulence genes in cecal colonization.Avian Dis. 2001 Jul-Sep;45(3):549-57. Avian Dis. 2001. PMID: 11569726
-
Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut.Vet Microbiol. 2008 Aug 25;130(3-4):285-97. doi: 10.1016/j.vetmic.2007.11.027. Epub 2007 Dec 3. Vet Microbiol. 2008. PMID: 18187272
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
-
Taking Control: Campylobacter jejuni Binding to Fibronectin Sets the Stage for Cellular Adherence and Invasion.Front Microbiol. 2020 Apr 9;11:564. doi: 10.3389/fmicb.2020.00564. eCollection 2020. Front Microbiol. 2020. PMID: 32328046 Free PMC article. Review.
Cited by
-
Autoinducer-2 production in Campylobacter jejuni contributes to chicken colonization.Appl Environ Microbiol. 2009 Jan;75(1):281-5. doi: 10.1128/AEM.01803-08. Epub 2008 Nov 14. Appl Environ Microbiol. 2009. PMID: 19011073 Free PMC article.
-
Refined functional carbohydrates reduce adhesion of Salmonella and Campylobacter to poultry epithelial cells in vitro.Poult Sci. 2020 Dec;99(12):7027-7034. doi: 10.1016/j.psj.2020.09.031. Epub 2020 Sep 25. Poult Sci. 2020. PMID: 33248619 Free PMC article.
-
Campylobacter growth rates in four different matrices: broiler caecal material, live birds, Bolton broth, and brain heart infusion broth.Infect Ecol Epidemiol. 2016 Apr 5;6:31217. doi: 10.3402/iee.v6.31217. eCollection 2016. Infect Ecol Epidemiol. 2016. PMID: 27052025 Free PMC article.
-
Quantifying bacterial evolution in the wild: A birthday problem for Campylobacter lineages.PLoS Genet. 2021 Sep 28;17(9):e1009829. doi: 10.1371/journal.pgen.1009829. eCollection 2021 Sep. PLoS Genet. 2021. PMID: 34582435 Free PMC article.
-
Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry.Front Microbiol. 2016 May 31;7:553. doi: 10.3389/fmicb.2016.00553. eCollection 2016. Front Microbiol. 2016. PMID: 27303366 Free PMC article. Review.
References
-
- Blaser, M. J., J. G. Wells, R. A. Feldman, R. A. Pollard, and J. R. Allen. 1983. Campylobacter enteritis in the United States. A multicenter study. Ann. Intern. Med. 98:360-365. - PubMed
-
- Doyle, M. P., and M. P. Jones. 1992. Food-borne transmission and antibiotic resistance of Campylobacter jejuni, p. 45-48. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni. Current status and future trends. American Society for Microbiology, Washington, DC.
-
- Fauchère, J.-L., M. Kervella, A. Rosenau, K. Mohanna, and M. Veron. 1989. Adhesion to HeLa cells of Campylobacter jejuni and C. coli outer membrane components. Res. Microbiol. 140:379-392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

