Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa bacteria by use of a microfluidic flow chamber
- PMID: 17293518
- PMCID: PMC1855618
- DOI: 10.1128/AEM.02649-06
Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa bacteria by use of a microfluidic flow chamber
Abstract
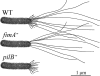
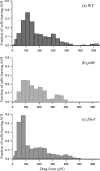
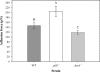
Xylella fastidiosa, a bacterium responsible for Pierce's disease in grapevines, possesses both type I and type IV pili at the same cell pole. Type IV pili facilitate twitching motility, and type I pili are involved in biofilm development. The adhesiveness of the bacteria and the roles of the two pili types in attachment to a glass substratum were evaluated using a microfluidic flow chamber in conjunction with pilus-defective mutants. The average adhesion force necessary to detach wild-type X. fastidiosa cells was 147 +/- 11 pN. Mutant cells possessing only type I pili required a force of 204 +/- 22 pN for removal, whereas cells possessing only type IV pili required 119 +/- 8 pN to dislodge these cells. The experimental results demonstrate that microfluidic flow chambers are useful and convenient tools for assessing the drag forces necessary for detaching bacterial cells and that with specific pilus mutants, the role of the pilus type can be further assessed.
Figures





References
-
- Batchelor, G. K. 1999. An introduction to fluid-dynamics, 2nd ed., p. 179-183. Cambridge University Press, Cambridge, United Kingdom.
-
- Chaudhury, M. K., and G. M. Whitesides. 1991. Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly(dimethylsiloxane) and their chemical derivatives. Langmuir 7:1013-1025.
-
- Davis, M. J., W. J. French, and N. W. Schaad. 1981. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Curr. Microbiol. 6:309-314.
-
- Galvani, C. D., Yaxin Li, T. J. Burr, and H. C. Hoch. 2007. Twitching motility among pathogenic Xylella fastidiosa isolates and the influence of bovine serum albumin on twitching-dependent colony fringe morphology. FEMS Microbiol. Lett. 268:202-208. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

