Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED
- PMID: 17293567
- PMCID: PMC1867337
- DOI: 10.1105/tpc.106.048272
Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED
Abstract
Chromatin remodeling is emerging as a central mechanism for patterning and differentiation in multicellular eukaryotes. SWI/SNF chromatin remodeling ATPases are conserved in the animal and plant kingdom and regulate transcriptional programs in response to endogenous and exogenous cues. In contrast with their metazoan orthologs, null mutants in two Arabidopsis thaliana SWI/SNF ATPases, BRAHMA (BRM) and SPLAYED (SYD), are viable, facilitating investigation of their role in the organism. Previous analyses revealed that syd and brm null mutants exhibit both similar and distinct developmental defects, yet the functional relationship between the two closely related ATPases is not understood. Another central question is whether these proteins act as general or specific transcriptional regulators. Using global expression studies, double mutant analysis, and protein interaction assays, we find overlapping functions for the two SWI/SNF ATPases. This partial diversification may have allowed expansion of the SWI/SNF ATPase regulatory repertoire, while preserving essential ancestral functions. Moreover, only a small fraction of all genes depends on SYD or BRM for expression, indicating that these SWI/SNF ATPases exhibit remarkable regulatory specificity. Our studies provide a conceptual framework for understanding the role of SWI/SNF chromatin remodeling in regulation of Arabidopsis development.
Figures

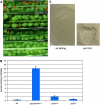
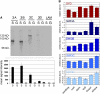

References
-
- Agresti, A. (1992). A survey of exact inference for contingency tables. Stat. Sci. 7 131–153.
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Angus-Hill, M.L., Schlichter, A., Roberts, D., Erdjument-Bromage, H., Tempst, P., and Cairns, B.R. (2001). A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell 7 741–751. - PubMed
-
- Blazquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404 889–892. - PubMed
-
- Breitling, R., Armengaud, P., Amtmann, A., and Herzyk, P. (2004). Rank products: A simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 573 83–92. - PubMed
NOTE ADDED IN PROOF
-
- Transcription profiling of Drosophila melanogaster pupae carrying dominant-negative mutations in SWI/SNF complex components revealed that 0.7 to 1.4% of all genes exhibited altered expression compared to wild-type pupae (Zraly et al., 2006). The extent of the alteration in gene expression is very similar to that which we observed in our study of expression changes in the SWI/SNF ATPase null mutant Arabidopsis thaliana seedlings compared to wild-type seedlings.
-
- Zraly, C.B., Middleton, F.A., and Dingwall, A.K. (2006). Hormone-response genes are direct in vivo regulatory targets of Brahma (SWI/SNF) complex function. J. Biol. Chem. 281 35305–35315. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

