Function, activity, and membrane targeting of cytosolic phospholipase A(2)zeta in mouse lung fibroblasts
- PMID: 17293613
- PMCID: PMC2678067
- DOI: 10.1074/jbc.M608458200
Function, activity, and membrane targeting of cytosolic phospholipase A(2)zeta in mouse lung fibroblasts
Abstract
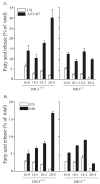
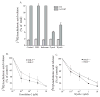
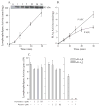
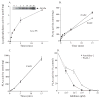
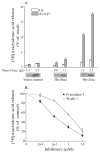
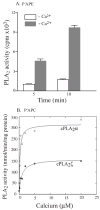
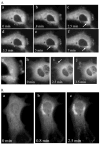
Group IVA cytosolic phospholipase A(2) (cPLA(2)alpha) initiates eicosanoid production; however, this pathway is not completely ablated in cPLA(2)alpha(-/-) lung fibroblasts stimulated with A23187 or serum. cPLA(2)alpha(+/+) fibroblasts preferentially released arachidonic acid, but A23187-stimulated cPLA(2)alpha(-/-) fibroblasts nonspecifically released multiple fatty acids. Arachidonic acid release from cPLA(2) alpha(-/-) fibroblasts was inhibited by the cPLA(2)alpha inhibitors pyrrolidine-2 (IC(50), 0.03 microM) and Wyeth-1 (IC(50), 0.1 microM), implicating another C2 domain-containing group IV PLA(2). cPLA(2) alpha(-/-) fibroblasts contain cPLA(2)beta and cPLA(2)zeta but not cPLA(2)epsilon or cPLA(2)delta. Purified cPLA(2)zeta exhibited much higher lysophospholipase and PLA(2) activity than cPLA(2)beta and was potently inhibited by pyrrolidine-2 and Wyeth-1, which did not inhibit cPLA(2)beta. In contrast to cPLA(2)beta, cPLA(2)zeta expressed in Sf9 cells mediated A23187-induced arachidonic acid release, which was inhibited by pyrrolidine-2 and Wyeth-1. cPLA(2)zeta exhibits specific activity, inhibitor sensitivity, and low micromolar calcium dependence similar to cPLA(2)alpha and has been identified as the PLA(2) responsible for calcium-induced fatty acid release and prostaglandin E(2) production from cPLA(2) alpha(-/-) lung fibroblasts. In response to ionomycin, EGFP-cPLA(2)zeta translocated to ruffles and dynamic vesicular structures, whereas EGFP-cPLA(2)alpha translocated to the Golgi and endoplasmic reticulum, suggesting distinct mechanisms of regulation for the two enzymes.
Figures

References
-
- Kudo I, Murakami M. Prostaglandins Other Lipid Mediat. 2002:68–69. 3–58. - PubMed
-
- Six DA, Dennis EA. Biochim Biophys Acta. 2000;1488:1–19. - PubMed
-
- Leslie CC. Biochem Cell Biol. 2004;82:1–17. - PubMed
-
- Ghosh M, Tucker DE, Burchett SA, Leslie CC. Prog Lipid Res. 2006;45:487–510. - PubMed
-
- Clark JD, Schievella AR, Nalefski EA, Lin LL. J Lipid Mediat Cell Signal. 1995;12:83–117. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

