Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors
- PMID: 17293861
- PMCID: PMC3027493
- DOI: 10.1038/nn1848
Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors
Abstract
Neurotransmitter release from mammalian sensory neurons is controlled by Ca(V)2.2 N-type calcium channels. N-type channels are a major target of neurotransmitters and drugs that inhibit calcium entry, transmitter release and nociception through their specific G protein-coupled receptors. G protein-coupled receptor inhibition of these channels is typically voltage-dependent and mediated by Gbetagamma, whereas N-type channels in sensory neurons are sensitive to a second G protein-coupled receptor pathway that inhibits the channel independent of voltage. Here we show that preferential inclusion in nociceptors of exon 37a in rat Cacna1b (encoding Ca(V)2.2) creates, de novo, a C-terminal module that mediates voltage-independent inhibition. This inhibitory pathway requires tyrosine kinase activation but not Gbetagamma. A tyrosine encoded within exon 37a constitutes a critical part of a molecular switch controlling N-type current density and G protein-mediated voltage-independent inhibition. Our data define the molecular origins of voltage-independent inhibition of N-type channels in the pain pathway.
Figures

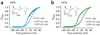
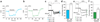



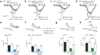

Comment in
-
Calcium channels diversify their signaling portfolio.Nat Neurosci. 2007 Mar;10(3):269-71. doi: 10.1038/nn0307-269. Nat Neurosci. 2007. PMID: 17318216 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

