Relaxin family peptide receptors--former orphans reunite with their parent ligands to activate multiple signalling pathways
- PMID: 17293890
- PMCID: PMC2013861
- DOI: 10.1038/sj.bjp.0707140
Relaxin family peptide receptors--former orphans reunite with their parent ligands to activate multiple signalling pathways
Abstract
The relaxin family peptides, although structurally closely related to insulin, act on a group of four G protein-coupled receptors now known as Relaxin Family Peptide (RXFP) Receptors. The leucine-rich repeat containing RXFP1 and RXFP2 and the small peptide-like RXFP3 and RXFP4 are the physiological targets for relaxin, insulin-like (INSL) peptide 3, relaxin-3 and INSL5, respectively. RXFP1 and RXFP2 have at least two binding sites--a high-affinity site in the leucine-rich repeat region of the ectodomain and a lower-affinity site in an exoloop of the transmembrane region. Although they respond to peptides that are structurally similar, RXFP3 and RXFP4 demonstrate distinct binding properties with relaxin-3 being the only peptide that can recognize these receptors in addition to RXFP1. Activation of RXFP1 or RXFP2 causes increased cAMP and the initial response for both receptors is the resultant of Gs-mediated activation and G(oB)-mediated inhibition of adenylate cyclase. With RXFP1, an additional delayed increase in cAMP involves betagamma subunits released from G(i3). In contrast, RXFP3 and RXFP4 inhibit adenylate cyclase and RXFP3 causes ERK1/2 phosphorylation. Drugs acting at RXFP1 have potential for the treatment of diseases involving tissue fibrosis such as cardiac and renal failure, asthma and scleroderma and may also be useful to facilitate embryo implantation. Activators of RXFP2 may be useful to treat cryptorchidism and infertility and inhibitors have potential as contraceptives. Studies of the distribution and function of RXFP3 suggest that it is a potential target for anti-anxiety and anti-obesity drugs.
Figures
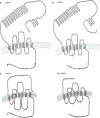

 indicates identical amino-acid residues,
indicates identical amino-acid residues,  shows highly homologous amino-acid residues and
shows highly homologous amino-acid residues and  indicates residues with some homology between all receptors.
indicates residues with some homology between all receptors.
 Indicates identical amino-acid residues,
Indicates identical amino-acid residues,  shows highly homologous amino-acid residues, and
shows highly homologous amino-acid residues, and  indicates residues with some homology between all receptors.
indicates residues with some homology between all receptors.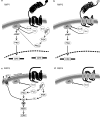
Similar articles
-
Relaxin family peptides and their receptors.Physiol Rev. 2013 Jan;93(1):405-80. doi: 10.1152/physrev.00001.2012. Physiol Rev. 2013. PMID: 23303914 Review.
-
Receptors for relaxin family peptides.Ann N Y Acad Sci. 2005 May;1041:61-76. doi: 10.1196/annals.1282.010. Ann N Y Acad Sci. 2005. PMID: 15956688 Review.
-
Membrane receptors: structure and function of the relaxin family peptide receptors.Mol Cell Endocrinol. 2010 May 14;320(1-2):1-15. doi: 10.1016/j.mce.2010.02.003. Epub 2010 Feb 6. Mol Cell Endocrinol. 2010. PMID: 20138959 Review.
-
International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides.Pharmacol Rev. 2006 Mar;58(1):7-31. doi: 10.1124/pr.58.1.9. Pharmacol Rev. 2006. PMID: 16507880 Review.
-
The electrostatic interactions of relaxin-3 with receptor RXFP4 and the influence of its B-chain C-terminal conformation.FEBS J. 2014 Jul;281(13):2927-36. doi: 10.1111/febs.12830. Epub 2014 May 27. FEBS J. 2014. PMID: 24802387
Cited by
-
Plasmodium falciparum suppresses the host immune response by inducing the synthesis of insulin-like peptides (ILPs) in the mosquito Anopheles stephensi.Dev Comp Immunol. 2015 Nov;53(1):134-44. doi: 10.1016/j.dci.2015.06.012. Epub 2015 Jul 9. Dev Comp Immunol. 2015. PMID: 26165161 Free PMC article.
-
Ligand-activated RXFP1 gene therapy ameliorates pressure overload-induced cardiac dysfunction.Mol Ther. 2021 Aug 4;29(8):2499-2513. doi: 10.1016/j.ymthe.2021.04.010. Epub 2021 Apr 9. Mol Ther. 2021. PMID: 33839322 Free PMC article.
-
Diverse functions of insulin-like 3 peptide.J Endocrinol. 2020 Oct 1;247(1):R1-R12. doi: 10.1530/JOE-20-0168. J Endocrinol. 2020. PMID: 32813485 Free PMC article. Review.
-
Insulin-like peptide 3 stimulates hemocytes to proliferate in anautogenous and facultatively autogenous mosquitoes.J Exp Biol. 2022 Mar 1;225(5):jeb243460. doi: 10.1242/jeb.243460. Epub 2022 Mar 8. J Exp Biol. 2022. PMID: 35129195 Free PMC article.
-
An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti.Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5716-21. doi: 10.1073/pnas.0800478105. Epub 2008 Apr 7. Proc Natl Acad Sci U S A. 2008. PMID: 18391205 Free PMC article.
References
-
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. - PubMed
-
- Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Human Reprod. 2001;7:799–809. - PubMed
-
- Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:324–334. - PubMed
-
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006a;58:7–31. - PubMed
-
- Bathgate RA, Lin F, Hanson NF, Otvos L, Jr, Guidolin A, Giannakis C, et al. Relaxin-3: improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry. 2006b;45:1043–1053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

