Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells
- PMID: 17295235
- PMCID: PMC2683254
- DOI: 10.1002/mc.20300
Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells
Abstract
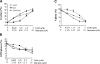
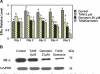
Although tamoxifen (TAM) is used for the front-line treatment and prevention of estrogen receptor-positive (ER+) breast tumors, nearly 40% of estrogen-dependent breast tumors do not respond to TAM treatment. Moreover, the positive response is usually of short duration, and most tumors eventually develop TAM-resistance. Overexpression of HER2 gene is associated with TAM-resistance of breast tumor, and suppression of HER2 expression enhances the TAM activity. Soy isoflavone genistein has been shown to have anti-cancer activities and suppress expression of HER2 and ERalpha. The objective of this study was to test the hypothesis that genistein may sensitize the response of ER+ and HER2-overexpressing breast cancer cells to TAM treatment. The combination treatment of TAM and genistein inhibited the growth of ER+/HER2-overexpressing BT-474 human breast cancer cells in a synergistic manner in vitro. Determination of cellular markers indicated that this synergistic inhibitory effect might be contributed in part from combined effects on cell-cycle arrest at G(1) phase and on induction of apoptosis. Further determination of the molecular markers showed that TAM and genistein combination synergistically induced BT-474 cell apoptosis in part by synergistic downregulation of the expression of survivin, one of the apoptotic effectors, and downregulation of EGFR, HER2, and ERalpha expression. Our research may provide a novel approach for the prevention and/or treatment of TAM insensitive/resistant human breast cancer, and warrants further in vivo studies to verify the efficacy of genistein and TAM combination on the growth of ER+/HER2-overexpressing breast tumors and to elucidate the in vivo mechanisms of synergistic actions.
(c) 2007 Wiley-Liss, Inc.
Figures




References
-
- Fisher B, Dignam J, Bryant J, et al. Five vs. more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. - PubMed
-
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: Twenty-eight years later. J Clin Oncol. 1995;13:513–529. - PubMed
-
- Muss HB. Endocrine therapy for advanced breast cancer: A review. Breast Cancer Res Treat. 1992;21:15–26. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of HER2/neu oncogene. Science. 1987;235:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

