Neurogranin binds to phosphatidic acid and associates to cellular membranes
- PMID: 17295609
- PMCID: PMC1868841
- DOI: 10.1042/BJ20061483
Neurogranin binds to phosphatidic acid and associates to cellular membranes
Abstract
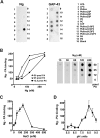
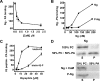
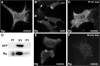
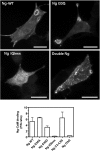
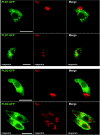
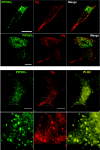
Neurogranin (Ng) is a 78-amino-acid-long protein concentrated at dendritic spines of forebrain neurons that is involved in synaptic plasticity through the regulation of CaM (calmodulin)-mediated signalling. Ng features a central IQ motif that mediates binding to CaM and is phosphorylated by PKC (protein kinase C). We have analysed the subcellular distribution of Ng and found that it associates to cellular membranes in rat brain. In vitro binding assays revealed that Ng selectively binds to PA (phosphatidic acid) and that this interaction is prevented by CaM and PKC phosphorylation. Using the peptide Ng-(29-47) and a mutant with an internal deletion (Ng-IQless), we have shown that Ng binding to PA and to cellular membranes is mediated by its IQ motif. Ng expressed in NIH-3T3 cells accumulates at peripheral regions of the plasma membrane and localizes at intracellular vesicles that can be clearly visualized following saponin permeabilization. This distribution was affected by PLD (phospholipase D) and PIP5K (phosphatidylinositol 4-phosphate 5-kinase) overexpression. Based on these results, we propose that Ng binding to PA may be involved in Ng accumulation at dendritic spines and that Ng could modulate PA signalling in the postsynaptic environment.
Figures


Similar articles
-
Activity-dependent translocation of neurogranin to neuronal nuclei.Biochem J. 2009 Dec 10;424(3):419-29. doi: 10.1042/BJ20091071. Biochem J. 2009. PMID: 19751214
-
Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity.IUBMB Life. 2010 Aug;62(8):597-606. doi: 10.1002/iub.357. IUBMB Life. 2010. PMID: 20665622 Review.
-
Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate.J Cell Biochem. 2007 Jan 1;100(1):112-28. doi: 10.1002/jcb.21027. J Cell Biochem. 2007. PMID: 16888807
-
Structural and dynamic characterization of a neuron-specific protein kinase C substrate, neurogranin.Biochemistry. 2003 May 6;42(17):5143-50. doi: 10.1021/bi0271751. Biochemistry. 2003. PMID: 12718558
-
[Neurogranin: a brain-specific protein].Sheng Li Ke Xue Jin Zhan. 2003 Apr;34(2):111-5. Sheng Li Ke Xue Jin Zhan. 2003. PMID: 12889141 Review. Chinese.
Cited by
-
Neurogranin-like immunoreactivity in the zebrafish brain during development.Brain Struct Funct. 2022 Nov;227(8):2593-2607. doi: 10.1007/s00429-022-02550-6. Epub 2022 Aug 26. Brain Struct Funct. 2022. PMID: 36018391 Free PMC article.
-
Neurogranin Expression Is Regulated by Synaptic Activity and Promotes Synaptogenesis in Cultured Hippocampal Neurons.Mol Neurobiol. 2019 Nov;56(11):7321-7337. doi: 10.1007/s12035-019-1593-3. Epub 2019 Apr 24. Mol Neurobiol. 2019. PMID: 31020616
-
Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation.PLoS One. 2012;7(7):e41275. doi: 10.1371/journal.pone.0041275. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848456 Free PMC article.
-
Neurogranin enhances synaptic strength through its interaction with calmodulin.EMBO J. 2009 Oct 7;28(19):3027-39. doi: 10.1038/emboj.2009.236. Epub 2009 Aug 27. EMBO J. 2009. PMID: 19713936 Free PMC article.
-
Alzheimer-associated cerebrospinal fluid fragments of neurogranin are generated by Calpain-1 and prolyl endopeptidase.Mol Neurodegener. 2018 Aug 29;13(1):47. doi: 10.1186/s13024-018-0279-z. Mol Neurodegener. 2018. PMID: 30157938 Free PMC article.
References
-
- Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17): identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J. Biol. Chem. 1991;266:229–237. - PubMed
-
- Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., Sutcliffe J. G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J. Neurosci. Res. 1990;26:397–408. - PubMed
-
- Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain: purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J. Biol. Chem. 1989;264:1824–1828. - PubMed
-
- Alvarez-Bolado G., Rodriguez-Sanchez P., Tejero-Diez P., Fairen A., Diez-Guerra F. J. Neurogranin in the development of the rat telencephalon. Neuroscience. 1996;73:565–580. - PubMed
-
- Yasuda H., Barth A. L., Stellwagen D., Malenka R. C. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

