A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor
- PMID: 17295905
- PMCID: PMC1802081
- DOI: 10.1186/1472-6807-7-6
A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor
Abstract
Background: Bone morphogenetic proteins (BMPs) are key regulators in the embryonic development and postnatal tissue homeostasis in all animals. Loss of function or dysregulation of BMPs results in severe diseases or even lethality. Like transforming growth factors beta (TGF-betas), activins, growth and differentiation factors (GDFs) and other members of the TGF-beta superfamily, BMPs signal by assembling two types of serine/threonine-kinase receptor chains to form a hetero-oligomeric ligand-receptor complex. BMP ligand receptor interaction is highly promiscuous, i.e. BMPs bind more than one receptor of each subtype, and a receptor bind various ligands. The activin type II receptors are of particular interest, since they bind a large number of diverse ligands. In addition they act as high-affinity receptors for activins but are also low-affinity receptors for BMPs. ActR-II and ActR-IIB therefore represent an interesting example how affinity and specificity might be generated in a promiscuous background.
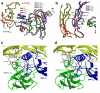
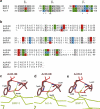
Results: Here we present the high-resolution structures of the ternary complexes of wildtype and a variant BMP-2 bound to its high-affinity type I receptor BMPR-IA and its low-affinity type II receptor ActR-IIB and compare them with the known structures of binary and ternary ligand-receptor complexes of BMP-2. In contrast to activin or TGF-beta3 no changes in the dimer architecture of the BMP-2 ligand occur upon complex formation. Functional analysis of the ActR-IIB binding epitope shows that hydrophobic interactions dominate in low-affinity binding of BMPs; polar interactions contribute only little to binding affinity. However, a conserved H-bond in the center of the type II ligand-receptor interface, which does not contribute to binding in the BMP-2 - ActR-IIB interaction can be mutationally activated resulting in a BMP-2 variant with high-affinity for ActR-IIB. Further mutagenesis studies were performed to elucidate the binding mechanism allowing us to construct BMP-2 variants with defined type II receptor binding properties.
Conclusion: Binding specificity of BMP-2 for its three type II receptors BMPR-II, Act-RII and ActR-IIB is encoded on single amino acid level. Exchange of only one or two residues results in BMP-2 variants with a dramatically altered type II receptor specificity profile, possibly allowing construction of BMP-2 variants that address a single type II receptor. The structure-/function studies presented here revealed a new mechanism, in which the energy contribution of a conserved H-bond is modulated by surrounding intramolecular interactions to achieve a switch between low- and high-affinity binding.
Figures
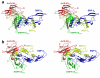


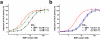
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

