Caspase-dependent proteolytic cleavage of STAT3alpha in ES cells, in mammary glands undergoing forced involution and in breast cancer cell lines
- PMID: 17295906
- PMCID: PMC1800902
- DOI: 10.1186/1471-2407-7-29
Caspase-dependent proteolytic cleavage of STAT3alpha in ES cells, in mammary glands undergoing forced involution and in breast cancer cell lines
Abstract
Background: The STAT (Signal Transducers and Activators of Transcription) transcription factor family mediates cellular responses to a wide range of cytokines. Activated STATs (particularly STAT3) are found in a range of cancers. Further, STAT3 has anti-apoptotic functions in a range of tumour cell lines. After observing a proteolytic cleavage in STAT3alpha close to a potential apoptotic caspase protease cleavage site we investigated whether STAT3alpha might be a caspase substrate.
Methods: STAT3alpha status was investigated in vitro in several cell systems:- HM-1 murine embryonic stem (ES) cells following various interventions; IOUD2 murine ES cells following induction to differentiate along neural or adipocyte lineages; and in a number of breast cancer cell lines. STAT3alpha status was also analysed in vivo in wild type murine mammary glands undergoing controlled, forced involution.
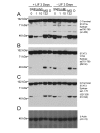
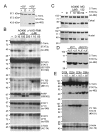
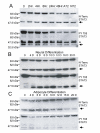
Results: Immunoblotting for STAT3alpha in HM-1 ES cell extracts detected amino and carboxy terminal species of approximately 48 kDa and 43 kDa respectively--which could be diminished dose-dependently by cell treatment with the nitric oxide (NO) donor drug sodium nitroprusside (SNP). UV irradiation of HM-1 ES cells triggered the STAT3alpha cleavage (close to a potential caspase protease cleavage site). Interestingly, the pan-caspase inhibitor z-Val-Ala-DL-Asp-fluoromethylketone (z-VAD-FMK) and the JAK2 tyrosine kinase inhibitor AG490 both inhibited cleavage dose-dependently, and cleavage was significantly lower in a heterozygous JAK2 knockout ES cell clone. STAT3alpha cleavage also occurred in vivo in normal murine mammary glands undergoing forced involution, coinciding with a pulse of phosphorylation of residue Y705 on full-length STAT3alpha. Cleavage also occurred during IOUD2 ES cell differentiation (most strikingly along the neural lineage) and in several human breast cancer cell lines, correlating strongly with Y705 phosphorylation.
Conclusion: This study documents a proteolytic cleavage of STAT3alpha into 48 kDa amino and 43 kDa carboxyl terminal fragments in a range of cell types. STAT3alpha cleavage occurs close to a potential caspase site, and can be inhibited dose-dependently by SNP, AG490 and z-VAD-FMK. The cleavage seems to be caspase-dependent and requires the phosphorylation of STAT3alpha at the Y705 residue. This highly regulated STAT3alpha cleavage may play an important role in modulating STAT3 transcriptional activity.
Figures

Similar articles
-
Phosphorylation and activation of STAT proteins by hypoxia in breast cancer cells.Breast. 2006 Apr;15(2):187-95. doi: 10.1016/j.breast.2005.05.005. Epub 2005 Aug 3. Breast. 2006. PMID: 16084091
-
WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells.Cancer Res. 2007 Dec 1;67(23):11291-9. doi: 10.1158/0008-5472.CAN-07-0593. Cancer Res. 2007. PMID: 18056455
-
p53 mediates a default programme of mammary gland involution in the absence of STAT3.Oncogene. 2005 Apr 28;24(19):3083-90. doi: 10.1038/sj.onc.1208512. Oncogene. 2005. PMID: 15735683
-
Caspases in cell survival, proliferation and differentiation.Cell Death Differ. 2007 Jan;14(1):44-55. doi: 10.1038/sj.cdd.4402047. Epub 2006 Oct 20. Cell Death Differ. 2007. PMID: 17053807 Review.
-
The Multifaceted Role of STAT3 in Mammary Gland Involution and Breast Cancer.Int J Mol Sci. 2018 Jun 7;19(6):1695. doi: 10.3390/ijms19061695. Int J Mol Sci. 2018. PMID: 29875329 Free PMC article. Review.
Cited by
-
Antiparkinson Drug Benztropine Suppresses Tumor Growth, Circulating Tumor Cells, and Metastasis by Acting on SLC6A3/DAT and Reducing STAT3.Cancers (Basel). 2020 Feb 24;12(2):523. doi: 10.3390/cancers12020523. Cancers (Basel). 2020. PMID: 32102440 Free PMC article.
-
The Wnt/β-catenin signaling pathway tips the balance between apoptosis and reprograming of cell fusion hybrids.Stem Cells. 2010 Nov;28(11):1940-9. doi: 10.1002/stem.515. Stem Cells. 2010. PMID: 20827748 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma/signal transducers and activators of transcription 5A pathway plays a key factor in adipogenesis of human bone marrow-derived stromal cells and 3T3-L1 preadipocytes.Stem Cells Dev. 2012 Feb 10;21(3):465-75. doi: 10.1089/scd.2010.0591. Epub 2011 Jun 15. Stem Cells Dev. 2012. PMID: 21542777 Free PMC article.
-
A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation.Chem Biol. 2007 Sep;14(9):1019-30. doi: 10.1016/j.chembiol.2007.07.016. Chem Biol. 2007. PMID: 17884634 Free PMC article.
-
Non-apoptotic function of caspases in a cellular model of hydrogen peroxide-associated colitis.J Cell Mol Med. 2013 Jul;17(7):901-13. doi: 10.1111/jcmm.12079. Epub 2013 Jun 7. J Cell Mol Med. 2013. PMID: 23742011 Free PMC article.
References
-
- Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

