Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation
- PMID: 17296732
- PMCID: PMC1899932
- DOI: 10.1128/MCB.02047-06
Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation
Abstract
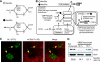
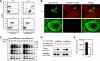
Sialic acid (Sia) is a family of acidic nine-carbon sugars that occupies the nonreducing terminus of glycan chains. Diversity of Sia is achieved by variation in the linkage to the underlying sugar and modification of the Sia molecule. Here we identified Sia-dependent epitope specificity for GL7, a rat monoclonal antibody, to probe germinal centers upon T cell-dependent immunity. GL7 recognizes sialylated glycan(s), the alpha2,6-linked N-acetylneuraminic acid (Neu5Ac) on a lactosamine glycan chain(s), in both Sia modification- and Sia linkage-dependent manners. In mouse germinal center B cells, the expression of the GL7 epitope was upregulated due to the in situ repression of CMP-Neu5Ac hydroxylase (Cmah), the enzyme responsible for Sia modification of Neu5Ac to Neu5Gc. Such Cmah repression caused activation-dependent dynamic reduction of CD22 ligand expression without losing alpha2,6-linked sialylation in germinal centers. The in vivo function of Cmah was analyzed using gene-disrupted mice. Phenotypic analyses showed that Neu5Gc glycan functions as a negative regulator for B-cell activation in assays of T-cell-independent immunization response and splenic B-cell proliferation. Thus, Neu5Gc is required for optimal negative regulation, and the reaction is specifically suppressed in activated B cells, i.e., germinal center B cells.
Figures





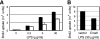

Similar articles
-
Functional evaluation of activation-dependent alterations in the sialoglycan composition of T cells.J Biol Chem. 2014 Jan 17;289(3):1564-79. doi: 10.1074/jbc.M113.523753. Epub 2013 Dec 2. J Biol Chem. 2014. PMID: 24297165 Free PMC article.
-
[Neu5Gc repression in germinal center B cells].Tanpakushitsu Kakusan Koso. 2008 Sep;53(12 Suppl):1630-5. Tanpakushitsu Kakusan Koso. 2008. PMID: 21089379 Review. Japanese. No abstract available.
-
Unmasking of CD22 Co-receptor on Germinal Center B-cells Occurs by Alternative Mechanisms in Mouse and Man.J Biol Chem. 2015 Dec 11;290(50):30066-77. doi: 10.1074/jbc.M115.691337. Epub 2015 Oct 27. J Biol Chem. 2015. PMID: 26507663 Free PMC article.
-
Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool.J Biol Chem. 1989 Dec 5;264(34):20216-23. J Biol Chem. 1989. PMID: 2684973
-
Possible Influences of Endogenous and Exogenous Ligands on the Evolution of Human Siglecs.Front Immunol. 2018 Dec 4;9:2885. doi: 10.3389/fimmu.2018.02885. eCollection 2018. Front Immunol. 2018. PMID: 30564250 Free PMC article. Review.
Cited by
-
Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms.Proc Natl Acad Sci U S A. 2019 Aug 6;116(32):16036-16045. doi: 10.1073/pnas.1902902116. Epub 2019 Jul 22. Proc Natl Acad Sci U S A. 2019. PMID: 31332008 Free PMC article.
-
Human-like NSG mouse glycoproteins sialylation pattern changes the phenotype of human lymphocytes and sensitivity to HIV-1 infection.BMC Immunol. 2019 Jan 7;20(1):2. doi: 10.1186/s12865-018-0279-3. BMC Immunol. 2019. PMID: 30616506 Free PMC article.
-
Review: The epic journey of sperm through the female reproductive tract.Animal. 2018 Jun;12(s1):s110-s120. doi: 10.1017/S1751731118000526. Epub 2018 Mar 19. Animal. 2018. PMID: 29551099 Free PMC article. Review.
-
Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen.J Immunol Methods. 2014 Feb;404:1-12. doi: 10.1016/j.jim.2013.11.026. Epub 2013 Dec 4. J Immunol Methods. 2014. PMID: 24316020 Free PMC article.
-
Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance.Trends Immunol. 2009 Oct;30(10):488-93. doi: 10.1016/j.it.2009.07.006. Epub 2009 Sep 18. Trends Immunol. 2009. PMID: 19766537 Free PMC article. Review.
References
-
- Alizadeh, A. A., M. B. Eisen, R. E. Davis, C. Ma, I. S. Lossos, A. Rosenwald, J. C. Boldrick, H. Sabet, T. Tran, X. Yu, J. I. Powell, L. Yang, G. E. Marti, T. Moore, J. Hudson, Jr., L. Lu, D. B. Lewis, R. Tibshirani, G. Sherlock, W. C. Chan, T. C. Greiner, D. D. Weisenburger, J. O. Armitage, R. Warnke, R. Levy, W. Wilson, M. R. Grever, J. C. Byrd, D. Botstein, P. O. Brown, and L. M. Staudt. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. - PubMed
-
- Baum, L. G., K. Derbin, N. L. Perillo, T. Wu, M. Pang, and C. Uittenbogaart. 1996. Characterization of terminal sialic acid linkages on human thymocytes. Correlation between lectin-binding phenotype and sialyltransferase expression. J. Biol. Chem. 271:10793-10799. - PubMed
-
- Blixt, O., B. E. Collins, I. M. Van Den Nieuwenhof, P. R. Crocker, and J. C. Paulson. 2003. Sialoside specificity of the Siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin associated glycoproteins. J. Biol. Chem. 278:31007-31019. - PubMed
-
- Buhl, A. M., and J. C. Cambier. 1997. Co-receptor and accessory regulation of B-cell antigen receptor signal transduction. Immunol. Rev. 160:127-138. - PubMed
-
- Cervenak, L., A. Magyar, R. Boja, and G. Laszlo. 2001. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol. Lett. 78:89-96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
