Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail
- PMID: 17296734
- PMCID: PMC1899918
- DOI: 10.1128/MCB.01315-06
Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail
Abstract
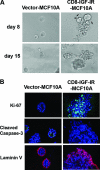
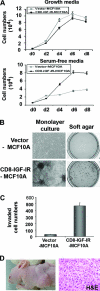
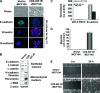
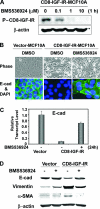
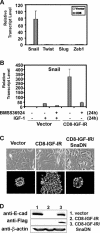
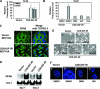
Type I insulin-like growth factor receptor (IGF-IR) can transform mouse fibroblasts; however, little is known about the transforming potential of IGF-IR in human fibroblasts or epithelial cells. We found that overexpression of a constitutively activated IGF-IR (CD8-IGF-IR) was sufficient to cause transformation of immortalized human mammary epithelial cells and growth in immunocompromised mice. Furthermore, CD8-IGF-IR caused cells to undergo an epithelial-to-mesenchymal transition (EMT) which was associated with dramatically increased migration and invasion. The EMT was mediated by the induction of the transcriptional repressor Snail and downregulation of E-cadherin. NF-kappaB was highly active in CD8-IGF-IR-MCF10A cells, and both increased levels of Snail and the EMT were partially reversed by blocking NF-kappaB or IGF-IR activity. This study places IGF-IR among a small group of oncogenes that, when overexpressed alone, can confer in vivo tumorigenic growth of MCF10A cells and indicates the hierarchy in the mechanism of IGF-IR-induced EMT.
Figures


Similar articles
-
NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2.Oncogene. 2007 Feb 1;26(5):711-24. doi: 10.1038/sj.onc.1209808. Epub 2006 Jul 24. Oncogene. 2007. PMID: 16862183
-
BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells.Clin Cancer Res. 2009 Jan 1;15(1):226-37. doi: 10.1158/1078-0432.CCR-08-0801. Clin Cancer Res. 2009. PMID: 19118050 Free PMC article.
-
Native type IV collagen induces an epithelial to mesenchymal transition-like process in mammary epithelial cells MCF10A.Int J Biochem Cell Biol. 2012 Dec;44(12):2194-203. doi: 10.1016/j.biocel.2012.08.018. Epub 2012 Sep 7. Int J Biochem Cell Biol. 2012. PMID: 22981734
-
Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions.Cells Tissues Organs. 2007;185(1-3):73-84. doi: 10.1159/000101306. Cells Tissues Organs. 2007. PMID: 17587811 Review.
-
Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry.Nitric Oxide. 2011 Jan 1;24(1):1-7. doi: 10.1016/j.niox.2010.10.001. Epub 2010 Oct 8. Nitric Oxide. 2011. PMID: 20933602 Review.
Cited by
-
Epithelial Mesenchymal Transition and Immune Response in Metaplastic Breast Carcinoma.Int J Mol Sci. 2021 Jul 9;22(14):7398. doi: 10.3390/ijms22147398. Int J Mol Sci. 2021. PMID: 34299016 Free PMC article. Review.
-
Proteomic Screening and Lasso Regression Reveal Differential Signaling in Insulin and Insulin-like Growth Factor I (IGF1) Pathways.Mol Cell Proteomics. 2016 Sep;15(9):3045-57. doi: 10.1074/mcp.M115.057729. Epub 2016 Jun 30. Mol Cell Proteomics. 2016. PMID: 27364358 Free PMC article.
-
BRD4 mediates NF-κB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation.Am J Physiol Lung Cell Mol Physiol. 2016 Dec 1;311(6):L1183-L1201. doi: 10.1152/ajplung.00224.2016. Epub 2016 Oct 28. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27793799 Free PMC article.
-
Combined effect of obesity and diabetes on early breast cancer outcome: a prospective observational study.Oncotarget. 2017 Dec 5;8(70):115709-115717. doi: 10.18632/oncotarget.22977. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383194 Free PMC article.
-
Inactivation of RARβ inhibits Wnt1-induced mammary tumorigenesis by suppressing epithelial-mesenchymal transitions.Nucl Recept Signal. 2014 Nov 4;12:e004. doi: 10.1621/nrs.12004. eCollection 2014. Nucl Recept Signal. 2014. PMID: 25422594 Free PMC article.
References
-
- Bakin, A. V., A. K. Tomlinson, N. A. Bhowmick, H. L. Moses, and C. L. Arteaga. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275:36803-36810. - PubMed
-
- Barbera, M. J., I. Puig, D. Dominguez, S. Julien-Grille, S. Guaita-Esteruelas, S. Peiro, J. Baulida, C. Franci, S. Dedhar, L. Larue, and A. Garcia de Herreros. 2004. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene 23:7345-7354. - PubMed
-
- Barrallo-Gimeno, A., and M. A. Nieto. 2005. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151-3161. - PubMed
-
- Baserga, R. 1999. The IGF-I receptor in cancer research. Exp. Cell Res. 253:1-6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
