Pharmacokinetics of saquinavir, atazanavir, and ritonavir in a twice-daily boosted double-protease inhibitor regimen
- PMID: 17296738
- PMCID: PMC1855477
- DOI: 10.1128/AAC.00854-06
Pharmacokinetics of saquinavir, atazanavir, and ritonavir in a twice-daily boosted double-protease inhibitor regimen
Abstract
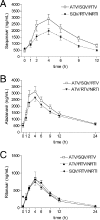
The objective of this study was to evaluate the pharmacokinetics of atazanavir (ATV), saquinavir (SQV), and ritonavir (RTV) in a boosted double-protease inhibitor (PI) therapy regimen without reverse transcriptase inhibitors (RTIs). The study design was as follows. Patients with limited RTI options received a PI combination of 300/100 mg ATV/RTV once daily and 1,000 mg SQV twice daily (group 1; n=49) without RTI comedication. The results were compared to the plasma concentrations of PIs of patients taking either 300 mg ATV/100 mg RTV once daily plus RTIs (group 2; n=72) or patients taking 1,000 mg SQV/100 mg RTV plus RTIs (group 3; n=90). The study methods were as follows. Patients were given a 12/24-h pharmacokinetic assessment at steady state. Drug concentrations were measured by liquid chromatography-tandem mass spectrometry. The minimum and maximum concentrations (Cmin and Cmax), area under the concentration-time curve under steady-state conditions (AUCss), elimination half-life, time of maximum concentration and lag time were subject to statistical analysis. The results show that patients treated with ATV/SQV/RTV exhibited significantly high SQV concentrations and moderate enhancement of the AUCss of ATV in comparison to those of patients of the control groups: for SQV in groups 1 and 3, the geometric mean (GM) of the AUCss was 22,794 versus 15,759 ng.h/ml (GM ratio [GMR]=1.45; P<0.05), the GM of the Cmax was 3,257 versus 2,331 ng/ml (GMR=1.40; P<0.05), and the GM of the Cmin was 438 versus 437 ng/ml (GMR=1.00); for ATV in groups 1 and 2, the GM of the AUCss was 39,154 versus 33,626 ng.h/ml (GMR=1.16), the GM of the Cmax was 3,488 versus 2,924 ng/ml (GMR=1.20), and the GM of the Cmin was 515 versus 428 ng/ml (GMR=1.21). RTV levels were comparable for all groups. A subgroup analysis detected only marginal differences in ATV plasma exposure if combined with tenofovir-disoproxilfumarate and without it. We conclude that our pharmacokinetic results support the use of a boosted double-PI regimen of ATV/SQV/RTV as a treatment option for patients who need antiretroviral therapy without RTIs.
Figures
Similar articles
-
The safety, efficacy, and pharmacokinetic profile of a switch in antiretroviral therapy to saquinavir, ritonavir, and atazanavir alone for 48 weeks and a switch in the saquinavir formulation.Clin Infect Dis. 2007 Jun 1;44(11):1475-83. doi: 10.1086/517507. Epub 2007 Apr 18. Clin Infect Dis. 2007. PMID: 17479946 Clinical Trial.
-
Pharmacokinetics of saquinavir with atazanavir or low-dose ritonavir administered once daily (ASPIRE I) or twice daily (ASPIRE II) in seronegative volunteers.J Clin Pharmacol. 2007 Feb;47(2):201-8. doi: 10.1177/0091270006296763. J Clin Pharmacol. 2007. PMID: 17244771
-
Steady-state pharmacokinetics of atazanavir given alone or in combination with saquinavir hard-gel capsules or amprenavir in HIV-1-infected patients.Eur J Clin Pharmacol. 2005 Aug;61(7):545-9. doi: 10.1007/s00228-005-0966-x. Epub 2005 Jul 23. Eur J Clin Pharmacol. 2005. PMID: 16041598
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
-
Saquinavir: a review of its use in boosted regimens for treating HIV infection.Drugs. 2003;63(12):1299-324. doi: 10.2165/00003495-200363120-00007. Drugs. 2003. PMID: 12790697 Review.
Cited by
-
Decrease of atazanavir and lopinavir plasma concentrations in a boosted double human immunodeficiency virus protease inhibitor salvage regimen.Antimicrob Agents Chemother. 2008 Jun;52(6):2273-5. doi: 10.1128/AAC.01565-07. Epub 2008 Apr 14. Antimicrob Agents Chemother. 2008. PMID: 18411323 Free PMC article.
-
Population pharmacokinetics of ritonavir-boosted atazanavir in HIV-infected patients and healthy volunteers.J Antimicrob Chemother. 2009 Jun;63(6):1233-43. doi: 10.1093/jac/dkp102. Epub 2009 Mar 28. J Antimicrob Chemother. 2009. PMID: 19329800 Free PMC article.
-
Concentration-dependent effects and intracellular accumulation of HIV protease inhibitors in cultured CD4 T cells and primary human lymphocytes.J Antimicrob Chemother. 2010 May;65(5):906-16. doi: 10.1093/jac/dkq082. Epub 2010 Mar 17. J Antimicrob Chemother. 2010. PMID: 20237075 Free PMC article.
-
Key Pharmacokinetic Essentials of Fixed-Dosed Combination Products: Case Studies and Perspectives.Clin Pharmacokinet. 2018 Apr;57(4):419-426. doi: 10.1007/s40262-017-0589-2. Clin Pharmacokinet. 2018. PMID: 28791593 Review.
-
Double-boosted protease inhibitor antiretroviral regimens: what role?Drugs. 2008;68(16):2257-67. doi: 10.2165/0003495-200868160-00001. Drugs. 2008. PMID: 18973392 Review.
References
-
- Barrios, A., A. Rendon, O. Rios, L. Martin-Carbonero, D. Gonzalez de Requena, O. Gallego, L. Valer, I. Maida, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2004. Atazanavir plasma levels associated with efficacy and safety in protease inhibitor-experienced HIV-infected patients, abstr. 606. Eleventh Conf. Retrovir. Oppor. Infect., San Francisco, CA.
-
- Bittner, B., M. Riek, B. Holmes, and S. Grange. 2005. Saquinavir 500 mg film-coated tablets demonstrate bioequivalence to saquinavir 200 mg hard capsules when boosted with twice-daily ritonavir in healthy volunteers. Antivir. Ther. 10:803-810. - PubMed
-
- Boffito, M., M. Kurowski, G. Kruse, A. Hill, A. A. Benzie, M. Nelson, G. Moyle, B. Gazzard, and A. Pozniak. 2004. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir boosted once-daily regimen. AIDS 18:1291-1297. - PubMed
-
- Boffito, M., D. Maitland, and A. Pozniak. 2006. Practical perspectives on the use of tipranavir in combination with other medications: lessons learned from pharmacokinetic studies. J. Clin. Pharmacol. 46:130-139. - PubMed
-
- Boffito, M., D. Maitland, Y. Samarasinghe, and A. Pozniak. 2005. The pharmacokinetics of HIV protease inhibitor combinations. Curr. Opin. Infect. Dis. 18:1-7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

