The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells
- PMID: 17296786
- PMCID: PMC2118715
- DOI: 10.1084/jem.20061338
The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells
Abstract
The Wiskott-Aldrich syndrome, a primary human immunodeficiency, results from defective expression of the hematopoietic-specific cytoskeletal regulator Wiskott-Aldrich syndrome protein (WASP). Because CD4(+)CD25(+)Foxp3(+) naturally occurring regulatory T (nTreg) cells control autoimmunity, we asked whether colitis in WASP knockout (WKO) mice is associated with aberrant development/function of nTreg cells. We show that WKO mice have decreased numbers of CD4(+)CD25(+)Foxp3(+) nTreg cells in both the thymus and peripheral lymphoid organs. Moreover, we demonstrate that WKO nTreg cells are markedly defective in both their ability to ameliorate the colitis induced by the transfer of CD45RB(hi) T cells and in functional suppression assays in vitro. Compared with wild-type (WT) nTreg cells, WKO nTreg cells show significantly impaired homing to both mucosal (mesenteric) and peripheral sites upon adoptive transfer into WT recipient mice. Suppression defects may be independent of antigen receptor-mediated actin rearrangement because both WT and WKO nTreg cells remodeled their actin cytoskeleton inefficiently upon T cell receptor stimulation. Preincubation of WKO nTreg cells with exogenous interleukin (IL)-2, combined with antigen receptor-mediated activation, substantially rescues the suppression defects. WKO nTreg cells are also defective in the secretion of the immunomodulatory cytokine IL-10. Overall, our data reveal a critical role for WASP in nTreg cell function and implicate nTreg cell dysfunction in the autoimmunity associated with WASP deficiency.
Figures



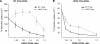



References
-
- Dupuis-Girod, S., J. Medioni, E. Haddad, P. Quartier, M. Cavazzana-Calvo, F. Le Deist, G. de Saint Basile, J. Delaunay, K. Schwarz, J.L. Casanova, et al. 2003. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 111:e622–e627. - PubMed
-
- Cannon, J.L., and J.K. Burkhardt. 2004. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173:1658–1662. - PubMed
-
- Badour, K., J. Zhang, F. Shi, M.K. McGavin, V. Rampersad, L.A. Hardy, D. Field, and K.A. Siminovitch. 2003. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 18:141–154. - PubMed
-
- Dupre, L., A. Aiuti, S. Trifari, S. Martino, P. Saracco, C. Bordignon, and M.G. Roncarolo. 2002. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 17:157–166. - PubMed
-
- Sasahara, Y., R. Rachid, M.J. Byrne, M.A. de la Fuente, R.T. Abraham, N. Ramesh, and R.S. Geha. 2002. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell. 10:1269–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

