Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells
- PMID: 17296787
- PMCID: PMC2118718
- DOI: 10.1084/jem.20061604
Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells
Abstract
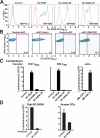
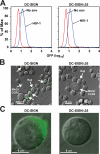
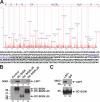
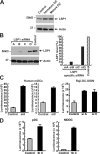
Dendritic cells (DCs) capture and internalize human immunodeficiency virus (HIV)-1 through C-type lectins, including DC-SIGN. These cells mediate efficient infection of T cells by concentrating the delivery of virus through the infectious synapse, a process dependent on the cytoplasmic domain of DC-SIGN. Here, we identify a cellular protein that binds specifically to the cytoplasmic region of DC-SIGN and directs internalized virus to the proteasome. This cellular protein, leukocyte-specific protein 1 (LSP1), was defined biochemically by immunoprecipitation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. LSP1 is an F-actin binding protein involved in leukocyte motility and found on the cytoplasmic surface of the plasma membrane. LSP1 interacted specifically with DC-SIGN and other C-type lectins, but not the inactive mutant DC-SIGNDelta35, which lacks a cytoplasmic domain and shows altered virus transport in DCs. LSP1 diverts HIV-1 to the proteasome. Down-regulation of LSP1 with specific small interfering RNAs in human DCs enhanced HIV-1 transfer to T cells, and bone marrow DCs from lsp1(-/-) mice also showed an increase in transfer of HIV-1(BaL) to a human T cell line. Proteasome inhibitors increased retention of viral proteins in lsp1(+/+) DCs, and substantial colocalization of virus to the proteasome was observed in wild-type compared with LSP1-deficient cells. Collectively, these data suggest that LSP1 protein facilitates virus transport into the proteasome after its interaction with DC-SIGN through its interaction with cytoskeletal proteins.
Figures

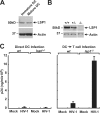
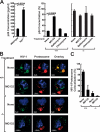
References
-
- Steinman, R.M., and K. Inaba. 1999. Myeloid dendritic cells. J. Leukoc. Biol. 66:205–208. - PubMed
-
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - PubMed
-
- Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. - PubMed
-
- Chesnut, R.W., and H.M. Grey. 1985. Antigen presenting cells and mechanisms of antigen presentation. Crit. Rev. Immunol. 5:263–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

