Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity
- PMID: 17296788
- PMCID: PMC2118729
- DOI: 10.1084/jem.20061737
Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity
Abstract
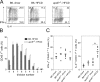
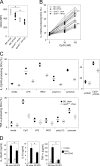
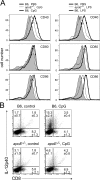
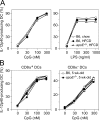
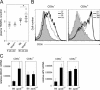
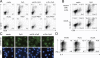
Environmental factors, including diet, play a central role in influencing the balance of normal immune homeostasis; however, many of the cellular mechanisms maintaining this balance remain to be elucidated. Using mouse models of genetic and high-fat/cholesterol diet-induced dyslipidemia, we examined the influence of dyslipidemia on T cell and dendritic cell (DC) responses in vivo and in vitro. We show that dyslipidemia inhibited Toll-like receptor (TLR)-induced production of proinflammatory cytokines, including interleukin (IL)-12, IL-6, and tumor necrosis factor-alpha, as well as up-regulation of costimulatory molecules by CD8alpha(-) DCs, but not by CD8alpha(+) DCs, in vivo. Decreased DC activation profoundly influenced T helper (Th) cell responses, leading to impaired Th1 and enhanced Th2 responses. As a consequence of this immune modulation, host resistance to Leishmania major was compromised. We found that oxidized low-density lipoprotein (oxLDL) was the key active component responsible for this effect, as it could directly uncouple TLR-mediated signaling on CD8alpha(-) myeloid DCs and inhibit NF-kappaB nuclear translocation. These results show that a dyslipidemic microenvironment can directly interfere with DC responses to pathogen-derived signals and skew the development of T cell-mediated immunity.
Figures

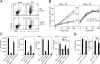
Similar articles
-
Invariant NKT cells preferentially modulate the function of CD8 alpha+ dendritic cell subset in inducing type 1 immunity against infection.J Immunol. 2010 Feb 15;184(4):2095-106. doi: 10.4049/jimmunol.0901348. Epub 2010 Jan 20. J Immunol. 2010. PMID: 20089704
-
Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori.Gastroenterology. 2007 Jul;133(1):150-163.e3. doi: 10.1053/j.gastro.2007.04.071. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17631139
-
A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity.J Immunol. 2011 Jun 1;186(11):6287-95. doi: 10.4049/jimmunol.1004036. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525388
-
Controlling the Toll road to dendritic cell polarization.J Leukoc Biol. 2004 May;75(5):721-30. doi: 10.1189/jlb.1003482. Epub 2004 Jan 14. J Leukoc Biol. 2004. PMID: 14726500 Review.
-
Dendritic cells from bench to bedside and back.Immunol Lett. 2009 Feb 21;122(2):128-30. doi: 10.1016/j.imlet.2008.11.017. Epub 2008 Dec 31. Immunol Lett. 2009. PMID: 19121337 Review.
Cited by
-
Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer.Sci Rep. 2015 Apr 29;5:9613. doi: 10.1038/srep09613. Sci Rep. 2015. PMID: 25923834 Free PMC article.
-
Predicting dyslipidemia after liver transplantation: A significant role of recipient metabolic inflammation profile.World J Gastroenterol. 2020 May 21;26(19):2374-2387. doi: 10.3748/wjg.v26.i19.2374. World J Gastroenterol. 2020. PMID: 32476799 Free PMC article.
-
Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases.Endocr Rev. 2012 Feb;33(1):71-108. doi: 10.1210/er.2011-0013. Epub 2012 Jan 12. Endocr Rev. 2012. PMID: 22240242 Free PMC article. Review.
-
Batf3-dependent CD8α+ Dendritic Cells Aggravates Atherosclerosis via Th1 Cell Induction and Enhanced CCL5 Expression in Plaque Macrophages.EBioMedicine. 2017 Apr;18:188-198. doi: 10.1016/j.ebiom.2017.04.008. Epub 2017 Apr 6. EBioMedicine. 2017. PMID: 28411140 Free PMC article.
-
Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss.Clin Immunol. 2009 Nov;133(2):265-75. doi: 10.1016/j.clim.2009.07.011. Epub 2009 Aug 22. Clin Immunol. 2009. PMID: 19699688 Free PMC article.
References
-
- Kapsenberg, M.L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984–993. - PubMed
-
- Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389–400. - PMC - PubMed
-
- Sporri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163–170. - PubMed
-
- Hsieh, C.S., S.E. Macatonia, C.S. Tripp, S.F. Wolf, A. O'Garra, and K.M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 260:547–549. - PubMed
-
- Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M.K. Gately, J.A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553–1559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

