Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes
- PMID: 17296797
- PMCID: PMC2063986
- DOI: 10.1083/jcb.200604167
Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes
Abstract
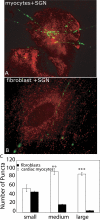
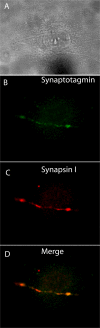
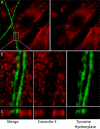
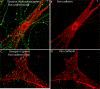
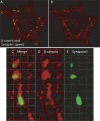
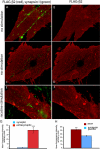
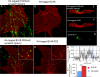
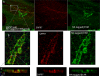
The sympathetic nervous system regulates cardiac function through the activation of adrenergic receptors (ARs). beta(1) and beta(2)ARs are the primary sympathetic receptors in the heart and play different roles in regulating cardiac contractile function and remodeling in response to injury. In this study, we examine the targeting and trafficking of beta(1) and beta(2)ARs at cardiac sympathetic synapses in vitro. Sympathetic neurons form functional synapses with neonatal cardiac myocytes in culture. The myocyte membrane develops into specialized zones that surround contacting axons and contain accumulations of the scaffold proteins SAP97 and AKAP79/150 but are deficient in caveolin-3. The beta(1)ARs are enriched within these zones, whereas beta(2)ARs are excluded from them after stimulation of neuronal activity. The results indicate that specialized signaling domains are organized in cardiac myocytes at sites of contact with sympathetic neurons and that these domains are likely to play a role in the subtype-specific regulation of cardiac function by beta(1) and beta(2)ARs in vivo.
Figures

References
-
- Armour, J.A., and J.L. Ardell. 1994. Neurocardiology. Oxford University Press, New York, NY. 443 pp.
-
- Bauman, A.L., A.S. Goehring, and J.D. Scott. 2004. Orchestration of synaptic plasticity through AKAP signaling complexes. Neuropharmacology. 46:299–310. - PubMed
-
- Bernstein, D., G. Fajardo, M. Zhao, T. Urashima, J. Powers, G. Berry, and B.K. Kobilka. 2005. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am. J. Physiol. Heart Circ. Physiol. 289:H2441–H2449. - PubMed
-
- Betz, W.J., and G.S. Bewick. 1992. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 255:200–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

