Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells
- PMID: 17296927
- PMCID: PMC2151613
- DOI: 10.1085/jgp.200609658
Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells
Abstract
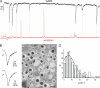
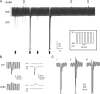
The release of gamma-aminobutyric acid (GABA) and ATP from rat beta cells was monitored using an electrophysiological assay based on overexpression GABA(A) or P2X2 receptor ion channels. Exocytosis of LDCVs, detected by carbon fiber amperometry of serotonin, correlated strongly (approximately 80%) with ATP release. The increase in membrane capacitance per ATP release event was 3.4 fF, close to the expected capacitance of an individual LDCV with a diameter of 0.3 microm. ATP and GABA were coreleased with serotonin with the same probability. Immunogold electron microscopy revealed that approximately 15% of the LDCVs contain GABA. Prespike "pedestals," reflecting exit of granule constituents via the fusion pore, were less frequently observed for ATP than for serotonin or GABA and the relative amplitude (amplitude of foot compared to spike) was smaller: in some cases the ATP-dependent pedestal was missing entirely. An inward tonic current, not dependent on glucose and inhibited by the GABA(A) receptor antagonist SR95531, was observed in beta cells in clusters of islet cells. Noise analysis indicated that it was due to the activity of individual channels with a conductance of 30 pS, the same as expected for individual GABA(A) Cl- channels with the ionic gradients used. We conclude that (a) LDCVs accumulate ATP and serotonin; (b) regulated release of GABA can be accounted for by exocytosis of a subset of insulin-containing LDCVs; (c) the fusion pore of LDCVs exhibits selectivity and compounds are differentially released depending on their chemical properties (including size); and (d) a glucose-independent nonvesicular form of GABA release exists in beta cells.
Figures

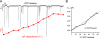

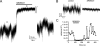
References
-
- Ämmälä, C., F.M. Ashcroft, and P. Rorsman. 1993. Calcium-independent potentiation of insulin release by cyclic AMP in single β cells. Nature. 363:356–358. - PubMed
-
- Aspinwall, C.A., L. Huang, J.R. Lakey, and R.T. Kennedy. 1999. Comparison of amperometric methods for detection of exocytosis from single pancreatic β cells of different species. Anal. Chem. 71:5551–5556. - PubMed
-
- Barg, S. 2003. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Pharmacol. Toxicol. 92:3–13. - PubMed
-
- Birnir, B., M.L. Tierney, N.P. Pillai, G.B. Cox, and P.W. Gage. 1995. Rapid desensitization of α1 β1 GABAA receptors expressed in Sf9 cells under optimized conditions. J. Membr. Biol. 148:193–202. - PubMed

