Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells
- PMID: 17296930
- PMCID: PMC1815231
- DOI: 10.1073/pnas.0611307104
Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells
Abstract
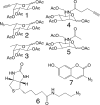
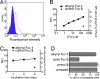
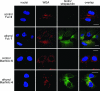
Developing tools for investigating the cellular activity of glycans will help to delineate the molecular basis for aberrant glycosylation in pathological processes such as cancer. Metabolic oligosaccharide engineering, which inserts sugar-reporting groups into cellular glycoconjugates, represents a powerful method for imaging the localization, trafficking, and dynamics of glycans and isolating them for glyco-proteomic analysis. Herein, we show that the alkyne-reporting group can be incorporated into cellular glycans. The alkyne group is a small, inert, bio-orthogonal handle that can be chemoselectively labeled by using the Cu(I) catalyzed [3 + 2] azide-alkyne cycloaddition, or click chemistry. Alkynyl sugar monomers, based on fucose (Fuc) and N-acetylmannosamine (ManNAc), were incorporated into fucosylated and sialylated glycans in several cancer cell lines, allowing for cell surface and intracellular visualization of glycoconjugates, as well as, observation of alkyne-bearing glycoproteins. Similarly to our previous results with an azido Fuc/alkynyl probe system, we demonstrated that click-activated fluorogenic probes are practical tools for efficiently and selectively labeling alkynyl-modified glycans. Because Fuc and sialic acid are terminal glycan residues with a notably increased presence in many tumors, we hope that our method will provide useful information about their roles in cancer and ultimately can be used for diagnostic and therapeutic purposes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Varki A, Cummings R, Esko JD, Freeze H, Hart GW, Marth J. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1999. pp. 1–635. - PubMed
-
- Axford JS. Biochim Biophys Acta. 1999;1455:219–229. - PubMed
-
- Dube DH, Bertozzi CR. Nat Rev Drug Discov. 2005;4:477–488. - PubMed
-
- Mackiewicz A, Mackiewicz K. Glycoconj J. 1995;12:241–247. - PubMed
-
- Meezan E, Wu HC, Black PH, Robbins PW. Biochemistry. 1969;8:2518–2524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

