A synthetic time-delay circuit in mammalian cells and mice
- PMID: 17296937
- PMCID: PMC1796999
- DOI: 10.1073/pnas.0606398104
A synthetic time-delay circuit in mammalian cells and mice
Abstract
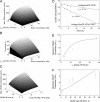
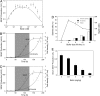
Time-delay circuitries in which a transcription factor processes independent input parameters can modulate NF-kappaB activation, manage quorum-sensing cross-talk, and control the circadian clock. We have constructed a synthetic mammalian gene network that processes four different input signals to control either immediate or time-delayed transcription of specific target genes. BirA-mediated ligation of biotin to a biotinylation signal-containing VP16 transactivation domain triggers heterodimerization of chimeric VP16 to a streptavidin-linked tetracycline repressor (TetR). At increasing biotin concentrations up to 20 nM, TetR-specific promoters are gradually activated (off to on, input signal 1), are maximally induced at concentrations between 20 nM and 10 microM, and are adjustably shut off at biotin levels exceeding 10 microM (on to off, input signal 2). These specific expression characteristics with a discrete biotin concentration window emulate a biotin-triggered bandpass filter. Removal of biotin from the culture environment (input signal 3) results in time-delayed transgene expression until the intracellular biotinylated VP16 pool is degraded. Because the TetR component of the chimeric transactivator retains its tetracycline responsiveness, addition of this antibiotic (input signal 4) overrides biotin control and immediately shuts off target gene expression. Biotin-responsive immediate, bandpass filter, and time-delay transcription characteristics were predicted by a computational model and have been validated in standard cultivation settings or biopharmaceutical manufacturing scenarios using trangenic CHO-K1 cell derivatives and have been confirmed in mice. Synthetic gene circuitries provide insight into structure-function correlations of native signaling networks and foster advances in gene therapy and biopharmaceutical manufacturing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Endy D. Nature. 2005;438:449–453. - PubMed
-
- Gibbs WW. Sci Am. 2004;290:74–81. - PubMed
-
- Hasty J, McMillen D, Collins JJ. Nature. 2002;420:224–230. - PubMed
-
- Sprinzak D, Elowitz MB. Nature. 2005;438:443–448. - PubMed
-
- Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ. Nature. 2006;439:856–860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

