Using polarization-shaped optical vortex traps for single-cell nanosurgery
- PMID: 17298009
- PMCID: PMC2519128
- DOI: 10.1021/nl0626784
Using polarization-shaped optical vortex traps for single-cell nanosurgery
Abstract
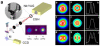
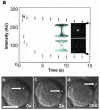
Single-cell nanosurgery and the ability to manipulate nanometer-sized subcellular structures with optical tweezers has widespread applications in biology but so far has been limited by difficulties in maintaining the functionality of the transported subcellular organelles. This difficulty arises because of the propensity of optical tweezers to photodamage the trapped object. To address this issue, this paper describes the use of a polarization-shaped optical vortex trap, which exerts less photodamage on the trapped particle than conventional optical tweezers, for carrying out single-cell nanosurgical procedures. This method is also anticipated to find broad use in the trapping of any nanoparticles that are adversely affected by high-intensity laser light.
Figures

Similar articles
-
Laser trapping of colloidal metal nanoparticles.ACS Nano. 2015;9(4):3453-69. doi: 10.1021/acsnano.5b00286. Epub 2015 Apr 1. ACS Nano. 2015. PMID: 25808609 Review.
-
Low-power nano-optical vortex trapping via plasmonic diabolo nanoantennas.Nat Commun. 2011 Dec 13;2:582. doi: 10.1038/ncomms1592. Nat Commun. 2011. PMID: 22158437
-
Application of plasmonic bowtie nanoantenna arrays for optical trapping, stacking, and sorting.Nano Lett. 2012 Feb 8;12(2):796-801. doi: 10.1021/nl203811q. Epub 2012 Jan 9. Nano Lett. 2012. PMID: 22208881
-
Numerical study of the properties of optical vortex array laser tweezers.Opt Express. 2013 Nov 4;21(22):26418-31. doi: 10.1364/OE.21.026418. Opt Express. 2013. PMID: 24216863
-
Towards biological applications of nanophotonic tweezers.Curr Opin Chem Biol. 2019 Dec;53:158-166. doi: 10.1016/j.cbpa.2019.09.008. Epub 2019 Oct 31. Curr Opin Chem Biol. 2019. PMID: 31678712 Free PMC article. Review.
Cited by
-
Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C. elegans.Curr Opin Neurobiol. 2009 Oct;19(5):561-7. doi: 10.1016/j.conb.2009.10.010. Epub 2009 Nov 5. Curr Opin Neurobiol. 2009. PMID: 19896831 Free PMC article. Review.
-
Laser selection significantly affects cell viability following single-cell nanosurgery.Photochem Photobiol. 2009 Sep-Oct;85(5):1218-24. doi: 10.1111/j.1751-1097.2009.00581.x. Epub 2009 Jun 22. Photochem Photobiol. 2009. PMID: 19558419 Free PMC article.
-
Defining Cell Cluster Size by Dielectrophoretic Capture at an Array of Wireless Electrodes of Several Distinct Lengths.Micromachines (Basel). 2019 Apr 23;10(4):271. doi: 10.3390/mi10040271. Micromachines (Basel). 2019. PMID: 31018537 Free PMC article.
-
Single-cell nanosurgery.Methods Mol Biol. 2013;991:139-48. doi: 10.1007/978-1-62703-336-7_14. Methods Mol Biol. 2013. PMID: 23546666 Free PMC article.
-
Nanonets Collect Cancer Secretome from Pericellular Space.PLoS One. 2016 Apr 21;11(4):e0154126. doi: 10.1371/journal.pone.0154126. eCollection 2016. PLoS One. 2016. PMID: 27100780 Free PMC article.
References
-
- Tu BP, Kudlicki A, Rowicka M, McKnight SL. Science. 2005;310(5751):1152–1158. - PubMed
-
- Hochedlinger K, Jaenisch R. Nature. 2006;441(7097):1061–1067. - PubMed
-
- Hochedlinger K, Rideout WM, Kyba M, Daley GQ, Blelloch R, Jaenisch R. Hematology Journal. 2004;5:S114–S117. - PubMed
-
- Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Optics Letters. 1986;11(5):288–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

