Transition-state analysis of S. pneumoniae 5'-methylthioadenosine nucleosidase
- PMID: 17298059
- PMCID: PMC2522316
- DOI: 10.1021/ja065082r
Transition-state analysis of S. pneumoniae 5'-methylthioadenosine nucleosidase
Abstract
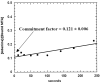
Kinetic isotope effects (KIEs) and computer modeling are used to approximate the transition state of S. pneumoniae 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN). Experimental KIEs were measured and corrected for a small forward commitment factor. Intrinsic KIEs were obtained for [1'-3H], [1'-14C], [2'-3H], [4'-3H], [5'-3H(2)], [9-15N] and [Me-3H(3)] MTAs. The intrinsic KIEs suggest an SN1 transition state with no covalent participation of the adenine or the water nucleophile. The transition state was modeled as a stable ribooxacarbenium ion intermediate and was constrained to fit the intrinsic KIEs. The isotope effects predicted a 3-endo conformation for the ribosyl oxacarbenium-ion corresponding to H1'-C1'-C2'-H2' dihedral angle of 70 degrees. Ab initio Hartree-Fock and DFT calculations were performed to study the effect of polarization of ribosyl hydroxyls, torsional angles, and the effect of base orientation on isotope effects. Calculations suggest that the 4'-3H KIE arises from hyperconjugation between the lonepair (n(p)) of O4' and the sigma* (C4'-H4') antibonding orbital owing to polarization of the 3'-hydroxyl by Glu174. A [methyl-3H(3)] KIE is due to hyperconjugation between np of sulfur and sigma* of methyl C-H bonds. The van der Waal contacts increase the 1'-3H KIE because of induced dipole-dipole interactions. The 1'-3H KIE is also influenced by the torsion angles of adjacent atoms and by polarization of the 2'-hydroxyl. Changing the virtual solvent (dielectric constant) does not influence the isotope effects. Unlike most N-ribosyltransferases, N7 of the leaving group adenine is not protonated at the transition state of S. pneumoniae MTAN. This feature differentiates the S. pneumoniae and E. coli transition states and explains the 10(3)-fold decrease in the catalytic efficiency of S. pneumoniae MTAN relative to that from E. coli.
Figures










Similar articles
-
Transition state structure of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli and its similarity to transition state analogues.Biochemistry. 2005 Sep 6;44(35):11647-59. doi: 10.1021/bi050863a. Biochemistry. 2005. PMID: 16128565
-
Transition-state structure of human 5'-methylthioadenosine phosphorylase.J Am Chem Soc. 2006 Nov 15;128(45):14691-6. doi: 10.1021/ja065419p. J Am Chem Soc. 2006. PMID: 17090056 Free PMC article.
-
Structure and inhibition of a quorum sensing target from Streptococcus pneumoniae.Biochemistry. 2006 Oct 31;45(43):12929-41. doi: 10.1021/bi061184i. Biochemistry. 2006. PMID: 17059210 Free PMC article.
-
Remote mutations alter transition-state structure of human purine nucleoside phosphorylase.Biochemistry. 2008 Feb 26;47(8):2565-76. doi: 10.1021/bi702133x. Biochemistry. 2008. PMID: 18281957
-
Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli.J Biol Chem. 2005 May 6;280(18):18265-73. doi: 10.1074/jbc.M414472200. Epub 2005 Mar 4. J Biol Chem. 2005. PMID: 15749708
Cited by
-
Enzymatic Transition States and Drug Design.Chem Rev. 2018 Nov 28;118(22):11194-11258. doi: 10.1021/acs.chemrev.8b00369. Epub 2018 Oct 18. Chem Rev. 2018. PMID: 30335982 Free PMC article. Review.
-
Screening for small molecule inhibitors of SAH nucleosidase using an SAH riboswitch.Anal Biochem. 2023 Apr 1;666:115047. doi: 10.1016/j.ab.2023.115047. Epub 2023 Jan 20. Anal Biochem. 2023. PMID: 36682579 Free PMC article.
-
Transition State Structure and Inhibition of Rv0091, a 5'-Deoxyadenosine/5'-methylthioadenosine Nucleosidase from Mycobacterium tuberculosis.ACS Chem Biol. 2016 Jun 17;11(6):1669-76. doi: 10.1021/acschembio.6b00144. Epub 2016 Apr 8. ACS Chem Biol. 2016. PMID: 27019223 Free PMC article.
-
Apparent Kinetic Isotope Effects for Multi-Step Steady-State Reactions.J Phys Chem B. 2025 Apr 10;129(14):3604-3609. doi: 10.1021/acs.jpcb.5c00561. Epub 2025 Mar 27. J Phys Chem B. 2025. PMID: 40146531 Free PMC article.
-
Transition-state variation in human, bovine, and Plasmodium falciparum adenosine deaminases.J Am Chem Soc. 2007 Jun 27;129(25):8008-17. doi: 10.1021/ja072122y. Epub 2007 May 31. J Am Chem Soc. 2007. PMID: 17536804 Free PMC article.
References
-
- Cleland WW. Arch Biochem Biophy. 2005;433:2–12. - PubMed
-
- Bennet AJ, Sinnot ML. J Am Chem Soc. 1986;108:7287–7294.
-
- Paneth B. Applications of Heavy Atom Isotope Effects. Synthesis and Applications of Isotopically Labeled Compounds. 1997
-
- Anet FAL, Basus VJ, Hewett APW, Saunders M. J Am Chem Soc. 1980;102:3945–3946.
-
- Lewis BE, Schramm VL. J Am Chem Soc. 2001;123:1327–1336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

